Hands-free Atalante exoskeleton in post-stroke gait and balance rehabilitation: a safety study
- PMID: 40221748
- PMCID: PMC11992748
- DOI: 10.1186/s12984-025-01621-z
Hands-free Atalante exoskeleton in post-stroke gait and balance rehabilitation: a safety study
Abstract
Background: Stroke often results in gait dysfunction, impairing daily activities and quality of life. Overground robotic exoskeletons hold promise for post-stroke rehabilitation. This study primarily aimed to assess the safety of hands-free Atalante exoskeleton training in post-stroke subjects, with a secondary aim to assess gait and balance.
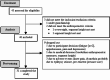
Methods: Forty subjects (10.2 ± 12.1 months post-stroke) with gait dysfunction (Functional Ambulation Category [FAC] score ≤ 3) underwent five training sessions over three weeks with a hands-free exoskeleton (Atalante, Wandercraft, France). Safety, the primary outcome, was evaluated by the number and severity of adverse events (AEs), judged by an independent clinical evaluation committee (CEC). A usability test was performed during the fifth training session followed by the exoskeleton use questionnaire. Gait and balance were assessed pre/post-training via walking capacity score (FAC), gait speed by 10-meter walk test (10MWT), walked distance by 6-minute walk test (6MWT), and balance by Berg Balance Scale (BBS). Spasticity was assessed with the Modified Ashworth scale. Anxiety and depression were quantified using the Hospital Anxiety and Depression Scale. Safety outcomes were analyzed using the Wilson, Lee and Dubin methods for proportions, and occurrence rates were computed. Within-group differences were compared using Wilcoxon, McNemar, and Friedman tests, with significance set at P < 0.05.
Results: Thirty-one subjects completed the training sessions, while nine discontinued. The study reported two serious adverse events (SAE) (vertigo, dysarthria) and six AEs, with the CEC concluding that no SAE was linked to the device/study procedure. The average AE rate per session was 2.5 ± 1.4%, including four events possibly linked to the device/study procedure (knee pain [n = 1], skin lacerations [n = 3]), classified as negligible or minor by the CEC. A high proportion (82.6%) successfully completed the usability test and reported satisfaction (90%) on the exoskeleton use questionnaire. For gait and balance, favorable changes were observed in FAC, 10MWT, 6MWT, and BBS scores Post-training (p < 0.05, respectively). Spasticity, anxiety, and depression remained unchanged.
Conclusions: This study indicates that the hands-free Atalante exoskeleton is safe, feasible, and well-tolerated for gait and balance rehabilitation in post-stroke subjects, warranting larger randomized controlled trials to assess its efficacy.
Trial registration: Evaluation of the Use of the Atalante Exoskeleton in Patients Presenting an Hemiplegia Due to Cerebrovascular Accident (INSPIRE) trial was registered at ClinicalTrials.gov (NCT04694001, registered on 20201231).
Keywords: Balance; Gait; Hands-free; Overground exoskeleton; Rehabilitation; Safety; Stroke.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the local ethics committee (CPP Ouest-IV, CE Hospitalo-Facultaire Saint Luc, and CNER, registered under ID RCB: 2020-A02437-32, B403202000079, and 202010/03, respectively). It was authorized by the French, Belgium and Luxembourg competent authorities (ANSM, AFMPS, and CNER). The study was promoted by the Wandercraft (CIP002) and registered in a public trial registry (Trial Registration: NCT04694001 ClinicalTrials.gov). All participants had agreed to participate and provided written informed consent. Consent for publication: Not applicable. Competing interests: Prof Thierry LEJEUNE, MD, PhD*; Stéphanie DEHEM, PhD; Jean-Gabriel PREVINAIRE, MD; Céline CUENOT, Pt; Jerome KAPS, PT; Bérénice PAUL, PT; Sergi SANZ PEREZ, PT, MSc; Fanny JUHEL, MD; Soultana TATSIDOU, MD, Jacques KERDRAON, MD have no conflict to declare. Vincent PÉAN, PhD received compensation by Wandercraft to perform the independent statistical analysis of the data. Dijana NUIC, PhD is employed by Wandercraft. Thierry DEBUGNE, MD is a speaker for Coloplast-AbbVie.
Figures


References
-
- Langhorne P, Coupar F, Pollock A. Motor recovery after stroke: a systematic review. Lancet Neurol. 2009;8(8):741–54. - PubMed
-
- Balaban B, Tok F. Gait disturbances in patients with stroke. PM&R. 2014;6(7):635–42. - PubMed
-
- Haute Autorité de Santé (HAS). Accident vasculaire cérébral: méthodes de rééducation de La Fonction motrice Chez l’adulte - Argumentaire scientifique. Has. 2012.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

