IRAK4 degrader in hidradenitis suppurativa and atopic dermatitis: a phase 1 trial
- PMID: 37957373
- PMCID: PMC10719089
- DOI: 10.1038/s41591-023-02635-7
IRAK4 degrader in hidradenitis suppurativa and atopic dermatitis: a phase 1 trial
Abstract
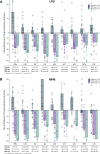
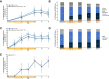
Toll-like receptor-driven and interleukin-1 (IL-1) receptor-driven inflammation mediated by IL-1 receptor-associated kinase 4 (IRAK4) is involved in the pathophysiology of hidradenitis suppurativa (HS) and atopic dermatitis (AD). KT-474 (SAR444656), an IRAK4 degrader, was studied in a randomized, double-blind, placebo-controlled phase 1 trial where the primary objective was safety and tolerability. Secondary objectives included pharmacokinetics, pharmacodynamics and clinical activity in patients with moderate to severe HS and in patients with moderate to severe AD. KT-474 was administered as a single dose and then daily for 14 d in 105 healthy volunteers (HVs), followed by dosing for 28 d in an open-label cohort of 21 patients. Degradation of IRAK4 was observed in HV blood, with mean reductions after a single dose of ≥93% at 600-1,600 mg and after 14 daily doses of ≥95% at 50-200 mg. In patients, similar IRAK4 degradation was achieved in blood, and IRAK4 was normalized in skin lesions where it was overexpressed relative to HVs. Reduction of disease-relevant inflammatory biomarkers was demonstrated in the blood and skin of patients with HS and patients with AD and was associated with improvement in skin lesions and symptoms. There were no drug-related infections. These results, from what, to our knowledge, is the first published clinical trial using a heterobifunctional degrader, provide initial proof of concept for KT-474 in HS and AD to be further confirmed in larger trials. ClinicalTrials.gov identifier: NCT04772885 .
© 2023. The Author(s).
Conflict of interest statement
A.G., V.C., A.D., S.A., R.K., K.S., A.M., C.K., B.E., V.M., E.K., K.S., E.K., R.B., D.C., X.Z., V.S., C.H., N.M., A.S. and J.A.G. are Kymera Therapeutics employees and may have stock or stock options. J.D. and H.R. are former Kymera Therapeutics employees and may have stock or stock options. J.C. is a full-time consultant working for Kymera Therapeutics. All other authors (L.A., G.A., S.B., H.S., B.J.F., P.L. and A.A.) are employees of their respective companies; they were investigators from clinical sites in the trial and received payment for their participation in the trial.
Figures







Comment in
-
PROTAC in clinic for autoimmune skin disease.Nat Rev Drug Discov. 2024 Jan;23(1):18. doi: 10.1038/d41573-023-00197-4. Nat Rev Drug Discov. 2024. PMID: 38049464 No abstract available.
References
-
- Dinarello CA. The IL-1 family of cytokines and receptors in rheumatic diseases. Nat. Rev. Rheumatol. 2019;15:612–632. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

