Multisystem Inflammatory Syndrome therapies in children (MISTIC): A randomized trial
- PMID: 36694613
- PMCID: PMC9852262
- DOI: 10.1016/j.conctc.2023.101060
Multisystem Inflammatory Syndrome therapies in children (MISTIC): A randomized trial
Abstract
Background: Multisystem Inflammatory Syndrome in Children (MIS-C), which occurs 2-6 weeks after initial exposure to SARS-CoV-2, was first identified in early 2020 when patients presented with fever and significant inflammation, often requiring management in the intensive care unit. To date, there has been no clinical trial to determine the most effective treatment. This study compares anti-inflammatory treatments that were selected based on current treatments for Kawasaki disease, a coronary artery vasculitis that shares many clinical features with MIS-C.
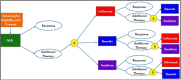
Methods: This randomized, comparative effectiveness trial of children with MIS-C uses the small N Sequential Multiple Assignment Randomized Trial (snSMART) design for rare diseases to compare multiple therapies within an individual. Study participants were treated first with intravenous immunoglobulin (IVIG), and if needed, subjects were then randomized to one of three additional treatments (steroids, anakinra, or infliximab). Participants were re-randomized to remaining treatments if they did not demonstrate clinical improvement.
Conclusion: This trial continues to enroll eligible participants to determine the most effective therapies in addition to IVIG and best order in which to use them to treat MIS-C.
Trial registration: NCT04898231.
Keywords: Anakinra; IVIG, Intravenous immunoglobulin; Infliximab; Multisystem inflammatory syndrome in children (MIS-C); Randomized clinical trial; Steroids; snSMART; snSMART, small N Sequential Multiple Assignment Randomized Trial.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Control, Centers for Disease Control and Prevention . 2020. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) 2020 May 14. [last accessed Sept 2022.
-
- Elias M.D., McCrindle B.W., Larios G., Choueiter N.F., Dahdah N., Harahsheh A.S., Jain S., Manlhiot C., Portman M.A., Raghuveer G., Giglia T.M., Dionne A., of the International Kawasaki Disease Registry Management of multisystem inflammatory syndrome in children associated with COVID-19: a survey from the international Kawasaki disease registry. CJC Open. 2020 Nov;2(6):632–640. doi: 10.1016/j.cjco.2020.09.004. Epub 2020 Sep 11. PMID: 32935083; PMCID: PMC7484693. - DOI - PMC - PubMed
-
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., Singh A.R., Li S., Tarquinio K.M., Jaggi P., Oster M.E., Zackai S.P., Gillen J., Ratner A.J., Walsh R.F., Fitzgerald J.C., Keenaghan M.A., Alharash H., Doymaz S., Clouser K.N., Giuliano J.S., Jr., Gupta A., Parker R.M., Maddux A.B., Havalad V., Ramsingh S., Bukulmez H., Bradford T.T., Smith L.S., Tenforde M.W., Carroll C.L., Riggs B.J., Gertz S.J., Daube A., Lansell A., Coronado Munoz A., Hobbs C.V., Marohn K.L., Halasa N.B., Patel M.M., Randolph A.G. Overcoming COVID-19 investigators; CDC COVID-19 response team. Multisystem inflammatory syndrome in U.S. Children and adolescents. N. Engl. J. Med. 2020 Jul 23;383(4):334–346. doi: 10.1056/NEJMoa2021680. Epub 2020 Jun 29. PMID: 32598831; PMCID: PMC7346765. - DOI - PMC - PubMed
-
- Kobayashi T., Saji T., Otani T., Takeuchi K., Nakamura T., Arakawa H., Kato T., Hara T., Hamaoka K., Ogawa S., Miura M., Nomura Y., Fuse S., Ichida F., Seki M., Fukazawa R., Ogawa C., Furuno K., Tokunaga H., Takatsuki S., Hara S., Morikawa A. RAISE study group investigators. Efficacy of immunoglobulin plus prednisolone for prevention of coronary artery abnormalities in severe Kawasaki disease (RAISE study): a randomised, open-label, blinded-endpoints trial. Lancet. 2012 Apr 28;379(9826):1613–1620. doi: 10.1016/S0140-6736(11)61930-2. Epub 2012 Mar 8. PMID: 22405251. - DOI - PubMed
-
- Tremoulet A.H., Jain S., Jaggi P., Jimenez-Fernandez S., Pancheri J.M., Sun X., Kanegaye J.T., Kovalchin J.P., Printz B.F., Ramilo O., Burns J.C. Infliximab for intensification of primary therapy for Kawasaki disease: a phase 3 randomised, double-blind, placebo-controlled trial. Lancet. 2014 May 17;383(9930):1731–1738. doi: 10.1016/S0140-6736(13)62298-9. Epub 2014 Feb 24. PMID: 24572997. - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

