Comparison of pharmacokinetics of a fixed-dose combination of atorvastatin/ezetimibe 5 mg/10 mg versus separate tablets in healthy subjects
- PMID: 40206873
- PMCID: PMC11976153
- DOI: 10.12793/tcp.2025.33.e5
Comparison of pharmacokinetics of a fixed-dose combination of atorvastatin/ezetimibe 5 mg/10 mg versus separate tablets in healthy subjects
Abstract
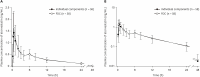
The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors are well-established treatment options for dyslipidemia. For patients not meeting low-density lipoprotein cholesterol targets with monotherapy, combination therapy with another lipid-lowering agent including ezetimibe, is recommended. This study compared the pharmacokinetics (PKs) and safety of a fixed-dose combination (FDC) of atorvastatin/ezetimibe 5 mg/10 mg with the individual components in healthy Korean subjects. A randomized, open-label, single-dose, 2-treatment, 2-sequence, crossover study was conducted in 60 healthy subjects. An FDC of atorvastatin/ezetimibe 5 mg/10 mg or the corresponding individual components was administered in the first period, and the alternative in the second period after a 14-day washout. Serial blood samples were collected up to 72 hours post-dose to calculate PK parameters such as maximum plasma concentration (Cmax) and the area under the plasma concentration-time curve to the last measurable concentration (AUClast). The geometric mean ratios (GMRs) and 90% confidence intervals (CIs) of the Cmax and AUClast for the atorvastatin and total ezetimibe were estimated compared to the individual components. Adverse events (AEs) and other safety variables were monitored to evaluate safety and tolerability profile. Sixty subjects were enrolled and 58 subjects completed the study. For atorvastatin, the GMRs (90% CIs) for Cmax and AUClast were 1.18 (1.04-1.33) and 1.04 (1.00-1.08), respectively, and the corresponding values were 1.37 (1.26-1.50) and 0.98 (0.93-1.03) for total ezetimibe. No clinically significant treatment-emergent AEs were observed with either formulations. The FDC of atorvastatin/ezetimibe 5 mg/10 mg was safe and showed similar exposure to those of the individual components.
Trial registration: ClinicalTrials.gov Identifier: NCT05202405.
Keywords: Atorvastatin; Drug Combinations; Dyslipidemia; Ezetimibe; Pharmacokinetics.
Copyright © 2025 Translational and Clinical Pharmacology.
Conflict of interest statement
Conflict of Interest: - Authors: Nothing to declare - Reviewers: Nothing to declare - Editors: Nothing to declare
Figures


References
-
- Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;140:e596–e646. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
