Evaluation of a second-generation intercostal extravascular implantable cardioverter defibrillator lead with a pectoral pulse generator for sensing, defibrillation, and anti-tachycardia pacing
- PMID: 40037337
- PMCID: PMC11928788
- DOI: 10.1093/europace/euaf044
Evaluation of a second-generation intercostal extravascular implantable cardioverter defibrillator lead with a pectoral pulse generator for sensing, defibrillation, and anti-tachycardia pacing
Abstract
Aims: Intercostal extravascular implantable cardioverter defibrillator (EV-ICD) leads may work better in contact with the pericardium thereby directing pacing and defibrillation energy towards excitable myocytes. We report 3-month safety and performance outcomes with a second-generation intercostal EV-ICD lead paired with standard, commercially available ICD pulse generators (PGs).
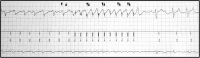
Methods and results: Subjects undergoing a transvenous ICD (TV-ICD) procedure received a concomitant intercostal EV-ICD lead system. The intercostal EV-ICD lead was connected sequentially to a PG in a left pectoral and then a left mid-axillary location. Extravascular ICD lead assessment included sensing and defibrillation of induced ventricular arrhythmias and pacing capture. The intercostal EV-ICD system was followed in a 'recording-only' mode and the control TV-ICD system in 'therapy delivery' mode to compare stored events. Devices were evaluated prior to hospital discharge, 2 weeks, 1 month, 2 months, and 3 months post-implant. Defibrillation testing was repeated prior to lead removal; 20/20 (100%) were successfully implanted (median implant time of 9 min). Two major lead complications were reported over a mean of 82 days: (i) lead movement and (ii) infection of both the TV-ICD and EV-ICD systems. Intraoperative pacing capture was achieved with the integrated bipolar configuration in 19 of 20 (95%) subjects. Pacing capture with the EV-ICD system was tolerated in all subjects, with over 90% feeling no pain after a 1-month recovery from the procedure. Induced VF episodes were sensed in all subjects and defibrillated successfully in 17 of 17 patients (100%) with a left mid-axillary PG and 19 of 20 patients (95%) with a left pectoral PG. Sensing and defibrillation were successful in 18 of 18 (100%) tested prior to lead removal.
Conclusion: In this pilot experience with a second-generation intercostal EV-ICD lead implantation, sensing and defibrillation of induced VF were successful when paired with a standard ICD PG from either a left mid-axillary or pectoral pocket.
Clinical trial registration: NCT number: NCT05791032; URL: https://clinicaltrials.gov/study/NCT05791032.
Keywords: Anterior mediastinum; Defibrillation; Extravascular; ICD; Intercostal.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: Authors have conflicts to disclose related to the manuscript as follows: M.C.B. has received honoraria and research grants from Boston Scientific and AtaCor Medical as well as owns equity in AtaCor Medical. S.K.D. and R.E.K. have received honoraria and research grants from Boston Scientific and Medtronic, consult for Abbott, Boston Scientific, and AtaCor Medical, and hold equity in AtaCor Medical. M.H., A.M., R.S., and D.S. are employees and shareholders of AtaCor Medical. A.E. has received research grants from AtaCor Medical.
Figures



References
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e73–189. - PubMed
-
- Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol 2005;28:926–32. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

