Impact of real-time glucose monitoring using FreeStyle Libre 3 on glycaemia in type 2 diabetes managed with basal insulin plus SGLT2 inhibitor and/or GLP-1 agonist: the FreeDM2 randomised controlled trial protocol
- PMID: 40233956
- PMCID: PMC12182046
- DOI: 10.1136/bmjopen-2024-090154
Impact of real-time glucose monitoring using FreeStyle Libre 3 on glycaemia in type 2 diabetes managed with basal insulin plus SGLT2 inhibitor and/or GLP-1 agonist: the FreeDM2 randomised controlled trial protocol
Abstract
Introduction: Effective management of type 2 diabetes mellitus (T2DM) consists of lifestyle modification and therapy optimisation. While glycaemic monitoring can be used as a tool to guide these changes, this can be challenging with self-monitoring of blood glucose (SMBG). The FreeStyle Libre 3 (FSL3) is a real-time continuous glucose monitoring (CGM) system designed to replace SMBG. The evidence for the benefit of CGM in people with T2DM on non-intensive insulin regimens is limited. This study aims primarily to assess the glycaemic impact of FSL3 in people with suboptimally controlled T2DM treated with basal-only insulin regimens plus sodium-glucose cotransporter-2 (SGLT-2) inhibitor and/or glucagon-like peptide (GLP)-1 agonist.
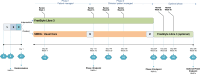
Methods and analysis: This is an open-label, multicentre, parallel design, randomised (2:1) controlled trial. Recruitment has been offered across 24 clinical centres in the UK and nationally through self-referral. Adults with T2DM treated with basal-only insulin regimens plus SGLT-2 inhibitor and/or GLP-1 agonist and with screening HbA1c from ≥59 mmol/mol to ≤97 mmol/mol are included. Eligible participants will be randomised to either FSL3 (intervention) for 32 weeks or continuation of SMBG (control). The study is split into two phases, each of 16 weeks duration: phase 1 consisting of self-management with basal-insulin self-titration and phase 2 where additional therapies may be initiated. Control group participants may subsequently enter an optional extension phase to receive FSL3. The primary endpoint is the difference between treatment groups in mean change from baseline in HbA1c at 16 weeks. Secondary outcomes include HbA1c at 32 weeks, CGM-based metrics, therapy changes, physical activity levels and psychosocial measures. An economic evaluation for costs and patient outcomes will be undertaken.
Ethics and dissemination: The study was approved by the Health Research Authority, Health and Care Research Wales and the West Midlands-Edgbaston Research Ethics Committee (reference: 23/WM/0092). Study results will be disseminated in peer-reviewed journals.
Trial registration number: NCT05944432.
Secondary identifying number: Identifier assigned by the sponsor: ADC-UK-PMS-22057.
Protocol version: Revision D. Dated, 13 December 2024.
Keywords: DIABETES & ENDOCRINOLOGY; Diabetes Mellitus, Type 2; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: EGW reports personal fees from Abbott, AstraZeneca, Dexcom, Eli Lilly, Embecta, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi, Ypsomed and research support from Abbott, Embecta, Insulet, Novo Nordisk and Sanofi. RA reports Institutional Research Grants and/or Honoraria/Educational Support/Consultancy from Abbott Diabetes Care, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Menarini Pharmaceuticals, NovoNordisk and Roche. PC reports personal fees from Abbott, Dexcom, Medtronic, Insulet, Roche, Novo Nordisk, Lilly, Sanofi, Vertex and Glooko. IC reports honoraria for educational and advisory board activity as well as research funding from Abbott. MLE reports personal fees from Abbott, Dexcom, Eli Lilly, Medtronic, Novo Nordisk, Pila Pharma, Zucara and research support from Abbott, Medtronic, Novo Nordisk and Sanofi. The University of Cambridge has received salary support for MLE from the NHS in the East of England through the Clinical Academic Reserve. AI reports research support from Dexcom Inc and honoraria from Astra Zeneca and Abbott. SK reports honoraria from Roche, Medtronic, Lilly and Boehringer Ingelheim. KBK reports research funding from Dexcom, Sanofi, Lilly, Insulet and Embecta, honoraria from Ascencia, Dexcom and Sanofi and is a founder and shareholder Spotlight-AQ. AL reports Institutional Research Grants and/or honoraria from Abbott Diabetes Care, Dexcom, Insulet, Eli Lilly, Medtronic, Menarini, Novo Nordisk and Sanofi and is a chair for Diabetes Technology Network-UK and an advisory board member for the EXTOD programme. TM reports personal fees and travel grants from AstraZeneca, Boehringer Ingelheim, Napp and Abbott. PN reports advisory board fees from Abbott, Eli Lilly and Insulet. SN reports personal fees from QUIN, Roche, Abbott Diabetes Care, Insulet and Astra Zeneca. GR reports personal fees from Abbott Diabetes Care, Sanofi Aventis and Eli Lilly. TS reports personal fees from Abbott, Rhythm Pharmaceuticals and research support from Abbott, Novo Nordisk, Pfizer and Novartis. HT reports consulting and speaking honoraria from Eli Lilly, Roche Diabetes and Dexcom and research support from Dexcom. TY reports research funding from AstraZeneca. LL reports personal fees from Abbott Diabetes Care, Dexcom, Insulet, Roche, Medtronic, Novo Nordisk and Sanofi Diabetes Care. YSC, RAE and PM report no relevant disclosures.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical