Study Protocol of a Prospective Phase 2 Study of Chlorophyllin for the Management of Brain Radionecrosis in Patients With Diffuse Glioma (CHROME)
- PMID: 40025673
- PMCID: PMC11872794
- DOI: 10.1002/cam4.70657
Study Protocol of a Prospective Phase 2 Study of Chlorophyllin for the Management of Brain Radionecrosis in Patients With Diffuse Glioma (CHROME)
Abstract
Introduction: Chlorophyllin (CHL) effectively decreases the side effects of radiotherapy (RT) by scavenging radiation-induced free radicals and reactive oxygen species in preclinical trials. This study aims to assess the efficacy of oral CHL for the treatment of brain radionecrosis in patients with diffuse glioma.
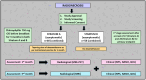
Methods: This is a phase 2 trial prospective, interventional study. Adults (> 18 years) with a histological diagnosis of diffuse glioma developing radionecrosis will be eligible for the study. Radionecrosis will be identified using standard imaging protocols with magnetic resonance imaging (MRI) with or without positron emission tomography (PET). Patients will be accrued in two strata: symptomatic (stratum A) and asymptomatic (stratum B). Chlorophyllin will be prescribed to all patients using a morning oral dose of 750 mg before breakfast for 3 months. In addition, participants in stratum A will be given a tapering dose of dexamethasone for 1 month, while stratum B will not be receiving any steroids. Imaging with an MRI brain protocol and PET scan will be planned at 1 month and MRI at 3 months after starting CHL. The primary endpoint is the clinical-radiological response at 1 month. Secondary endpoints include response at 3 months, biological responses, survival analysis, and quality-of-life scores. The total sample size is 118 (60 and 58 in stratum A and B, respectively), with one interim analysis planned.
Discussion: Radionecrosis leads to significant morbidity and is usually treated with corticosteroids, which can lead to several side effects from both acute and long-term use. Refractory radionecrosis requires treatment with bevacizumab or surgical resection. Chlorophyllin is a cheap, safe, and readily available phytopharmaceutical drug, which is being investigated in the phase 2 study and, if proven effective, can be considered an alternative for treating radionecrosis.
Trial registration: Clinical Trial Registry India (CTRI): CTRI/2023/08/056166; ClinicalTrials.gov: NCT06016452.
Keywords: chlorophyllin; diffuse glioma; glioblastoma; high‐grade glioma; radionecrosis.
© 2025 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Stupp R., Mason W. P., van den Bent M. J., et al., “Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma,” New England Journal of Medicine 352 (2005): 987–996. - PubMed
-
- Mohile N. A., Messersmith H., Gatson N. T., et al., “Therapy for Diffuse Astrocytic and Oligodendroglial Tumors in Adults: ASCO‐SNO Guideline,” Journal of Clinical Oncology 40 (2022): 403–426. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

