GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes
- PMID: 38184582
- PMCID: PMC10771648
- DOI: 10.1186/s12933-023-02118-6
GLP-1 receptor agonists-SGLT-2 inhibitors combination therapy and cardiovascular events after acute myocardial infarction: an observational study in patients with type 2 diabetes
Abstract
Background: Few studies explored the effect of the combination of glucose sodium-cotransporter-2 inhibitors (SGLT-2i) and glucagon-like peptide-1 receptor agonists (GLP-1RA) on the incidence of cardiovascular events in patients with type 2 diabetes (T2D) and acute myocardial infarction (AMI).
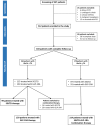
Methods: We recruited patients with T2D and AMI undergoing percutaneous coronary intervention, treated with either SGLT-2i or GLP-1RA for at least 3 months before hospitalization. Subjects with HbA1c < 7% at admission were considered in good glycemic control and maintained the same glucose-lowering regimen, while those with poor glycemic control (HbA1c ≥ 7%), at admission or during follow-up, were prescribed either a SGLT-2i or a GLP-1RA to obtain a SGLT-2i/GLP-1RA combination therapy. The primary outcome was the incidence of major adverse cardiovascular events (MACE) defined as cardiovascular death, re-acute coronary syndrome, and heart failure related to AMI during a 2-year follow-up. After 3 months, the myocardial salvage index (MSI) was assessed by single-photon emission computed tomography.
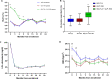
Findings: Of the 537 subjects screened, 443 completed the follow-up. Of these, 99 were treated with SGLT-2i, 130 with GLP-1RA, and 214 with their combination. The incidence of MACE was lower in the combination therapy group compared with both SGLT-2i and GLP-1RA treated patients, as assessed by multivariable Cox regression analysis adjusted for cardiovascular risk factors (HR = 0.154, 95% CI 0.038-0.622, P = 0.009 vs GLP-1RA and HR = 0.170, 95% CI 0.046-0.633, P = 0.008 vs SGLT-2i). The MSI and the proportion of patients with MSI > 50% was higher in the SGLT-2i/GLP-1RA group compared with both SGLT-2i and GLP-1RA groups.
Interpretation: The combination of SGLT-2i and GLP-1RA is associated with a reduced incidence of cardiovascular events in patients with T2D and AMI compared with either drug used alone, with a significant effect also on peri-infarcted myocardial rescue in patients without a second event. Trial registraition ClinicalTrials.gov ID: NCT06017544.
Keywords: Combination therapies; Diabetes algorithm; GLP-1 receptor agonists; Glucose-lowering drugs; Heart failure; MACE; Myocardial infarction; SGLT-2 inhibitors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Best JH, Hoogwerf BJ, Herman WH, et al. Risk of cardiovascular disease events in patients with type 2 diabetes prescribed the glucagon-like peptide 1 (GLP-1) receptor agonist exenatide twice daily or other glucose-lowering therapies: a retrospective analysis of the LifeLink database. Diabetes Care. 2011;34(1):90–95. doi: 10.2337/dc10-1393. - DOI - PMC - PubMed
-
- Palmer SC, Tendal B, Mustafa RA, et al. Sodium–glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. - DOI - PMC - PubMed
-
- Sattar N, Lee MMY, Kristensen SL, et al. Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of randomised trials. Lancet Diabetes Endocrinol. 2021;9(10):653–662. doi: 10.1016/S2213-8587(21)00203-5. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

