The European Larynx Organ Preservation Study [MK-3475-C44]
- PMID: 39239277
- PMCID: PMC11374655
- DOI: 10.3389/fonc.2024.1433238
The European Larynx Organ Preservation Study [MK-3475-C44]
Abstract
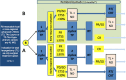
The European Larynx Organ Preservation Study (ELOS; NCT06137378) is a prospective, randomized, open-label, two-armed parallel group controlled, phase II multicenter larynx organ preservation (LOP) trial in locoregionally advanced (LA) stage III, IVA/B head and neck squamous cell carcinoma of the larynx or hypopharynx (LHSCC) amenable for total laryngectomy (TL) with PD-L1 expression within tumor tissue biopsy, calculated as CPS ≥ 1. Induction chemotherapy (IC) with docetaxel and cisplatin (TP) followed by radiation will be compared to TP plus PD-1 inhibition by pembrolizumab (MK-3475; 200 mg i.v. starting day 1 q3w for 17 cycles). After a short induction early response evaluation (ERE) 21 ± 3 days after the first cycle of IC (IC-1), responders achieving endoscopic estimated tumor surface shrinkage (ETSS) ≥30% will get an additional two cycles of IC followed by intensity-modulated radiotherapy 70-72 Gy (EQD2/α/β = 10) aiming at LOP. Nonresponders (ETSS < 30% or progressing disease) will receive TL and bilateral neck dissection followed by postoperative radiation or chemoradiation as recommended by the clinic's multidisciplinary tumor board. Pembrolizumab treatment will be continued in the intervention arm regardless of ETSS status after IC-1 in both responders and laryngectomized nonresponders, independent of subsequent decisions on adjuvant therapy after TL.
Clinical trial registration: clinicaltrials.gov, identifier NCT06137378.
Keywords: head and neck squamous cell carcinoma (HNSCC); immune checkpoint blockade PD-1:PD-L1 axis; inductionchemotherapy (IC); larynx and hypopharynx cancer (LHSCC); larynx organ function; larynx organ preservation (LOP); randomized controlled trial (RCT); total laryngectomy (TL).
Copyright © 2024 Wichmann, Wald, Pirlich, Napp, Münter, Asendorf, Tostmann, Vogt, Vogel, Meuret, Stoehr, Zebralla, Nicolay, Kuhnt, Hambsch, Guntinas-Lichius, Klußmann, Wiegand and Dietz.
Conflict of interest statement
Some of the authors disclosed potential conflicts of interest. GW discloses honoraria for lectures from Merck KGaA, outside the submitted work; OG-L declares financial Interests, Personal, Invited Speaker: Novartis, Merz, and MedEL; Financial Interests, Personal, Advisory Board: Merz; Financial Interests, Personal and Institutional, Research Grant: MedEL; Financial Interests, Personal and Institutional, Funding: MedEL. JK reports personal fees from Merck KGaA, outside the submitted work. SW declares remuneration for scientific presentations or participation on Advisory Boards for MSD, BMS, Merck Serono, Roche, AstraZeneca, Sanofi, and GSK. AD reports remuneration for scientific presentations and participation on Advisory Boards for Merck Serono, Roche, AstraZeneca, MSD, BMS, Sanofi, Norgine, Nanobiotix and GSK and research support from Roche, Merck Serono, and MSD. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
-
- Dietz A. ELOS - Induction chemotherapy with docetaxel and cisplatin followed by radiation compared to additional PD-1 inhibition in CPS ≥1 advanced laryngeal/hypopharyngeal cancer suitable for laryngectomy selected after early response evaluation (European Larynx Organ Preservation Study (ELOS) [MK-3475-C44], 2022-502751-61-00. European Medicines Agency (CTIS). The Netherlands: Amsterdam (2023) The Netherlands: Amsterdam. Available at: CinicalTrials.gov No.: NCT06137378.
-
- Dietz A, Wichmann G, Kuhnt T, Pfreundner L, Hagen R, Scheich M, et al. . Induction chemotherapy (IC) followed by radiotherapy (RT) versus cetuximab plus IC and RT in advanced laryngeal/hypopharyngeal cancer resectable only by total laryngectomy-final results of the larynx organ preservation trial DeLOS-II. Ann Oncol. (2018) 29:2105–14. doi: 10.1093/annonc/mdy332 - DOI - PubMed
-
- Wichmann G, Krüger A, Boehm A, Kolb M, Hofer M, Fischer M, et al. . Induction chemotherapy followed by radiotherapy for larynx preservation in advanced laryngeal and hypopharyngeal cancer: Outcome prediction after one cycle induction chemotherapy by a score based on clinical evaluation, computed tomography-based volumetry and 18F-FDG-PET/CT. Eur J Cancer. (2017) 72:144–55. doi: 10.1016/j.ejca.2016.11.013 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

