Effects of intravascular administration of mesenchymal stromal cells derived from Wharton's Jelly of the umbilical cord on systemic immunomodulation and neuroinflammation after traumatic brain injury (TRAUMACELL): study protocol for a multicentre randomised controlled trial
- PMID: 39740941
- PMCID: PMC11749534
- DOI: 10.1136/bmjopen-2024-091441
Effects of intravascular administration of mesenchymal stromal cells derived from Wharton's Jelly of the umbilical cord on systemic immunomodulation and neuroinflammation after traumatic brain injury (TRAUMACELL): study protocol for a multicentre randomised controlled trial
Abstract
Introduction: Traumatic brain injury (TBI) is one of the leading causes of death and disability worldwide. Treatments for TBI patients are limited and none has been shown to provide prolonged and long-term neuroprotective or neurorestorative effects. A growing body of evidence suggests a link between TBI-induced neuro-inflammation and neurodegenerative post-traumatic disorders. Consequently, new therapies triggering immunomodulation and promoting neurological recovery are the subject of major research efforts. We hypothesise that repeated intravenous treatment with mesenchymal stromal cells derived from Wharton's Jelly of the umbilical cord-derived mesenchymal stromal cells ((WJ-UC-MSC) may be associated with a significant decrease of post-TBI neuroinflammation and improvement of neurological status.
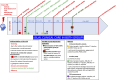
Methods and analysis: The TRAUMACELL trial is a prospective, national multicentre, phase III, superiority, double-arm comparative randomised (1:1) double-blinded clinical trial. Among patients aged between 18-50, with a severe TBI defined by a Glasgow score less than 12 (within the first 48 hours) with brain traumatic lesion on CT Scan and needing intracranial pressure monitoring, with no other significant organ trauma (abbreviated injury scale<2) and unresponsive to verbal commands after 5 days of sedation discontinuation, 68 will be randomly allocated to receive either WJ-UC-MSC solution or placebo, with three intravenous injections 1 week apart. The primary outcome is the [18F]-DPA-714 signal intensity in corpus callosum measured by dynamic positron emission tomography (PET)-MRI at 6 months after the last injection, blinded to the randomisation arm, to evaluate the post-traumatic neuro-inflammation.
Ethics and dissemination: The TRAUMACELL trial has been approved by an independent ethics committee (CPP SUD EST II) and French Medicines Agency (2023-504415-33-00) for all study centres. Participant recruitment will be starting in September 2024. Results will be published in international peer-reviewed medical journals.
Trial registration number: NCT06146062, first posted 24 November 2023 PROTOCOL VERSION IDENTIFIER: TRAUMACELL-V.2.0_20240102.
Keywords: Adult intensive & critical care; Brain Injuries; Clinical Trial; Mesenchymal Stem Cells; NUCLEAR MEDICINE; Neuroradiology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials