The Effect of Effort During a Resistance Exercise Session on Glycemic Control in Individuals Living With Prediabetes or Type 2 Diabetes: Protocol for a Crossover Randomized Controlled Trial
- PMID: 39499920
- PMCID: PMC11576611
- DOI: 10.2196/63598
The Effect of Effort During a Resistance Exercise Session on Glycemic Control in Individuals Living With Prediabetes or Type 2 Diabetes: Protocol for a Crossover Randomized Controlled Trial
Abstract
Background: Type 2 diabetes (T2D) is preceded by prediabetes, and these conditions place a great burden on patients and society. These conditions are significantly associated with poor glycemic control, which is improved by resistance exercise. It has been suggested that resistance exercise should be performed with a high degree of effort to improve glucose metabolism, but this is associated with negative psychological responses that might lead to lower long-term adherence.
Objective: This study aimed to investigate the effect of the degree of effort during a resistance exercise session on glycemic control and psychological responses in individuals living with prediabetes or T2D.
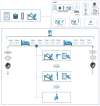
Methods: This study will be a crossover, 3-arm, randomized controlled trial. A total of 15 participants living with prediabetes or T2D will be thoroughly familiarized with 7 resistance exercises; afterward, they will perform 3 randomized experimental sessions, each lasting approximately 48 hours each, separated by at least 4 washout days. In 2 of these sessions, supervised resistance exercise will be performed, but the sessions will differ in the degree of effort in each set (high vs low) and will be equalized in terms of total weight lifted and session duration. For this, proximity to failure will be manipulated by changing the number of sets per exercise, the number of repetitions per set, and the resting interval between sets and exercises. Participants will also complete a sedentary (control) session, where they will not perform any exercise. In response to each session, psychological responses will be assessed (exertion, affect, enjoyment, self-efficacy, and discomfort). Glycemic control will be assessed by a continuous glucose monitoring device every 5 minutes, throughout the approximately 48 hours of each experimental session. Food and drink will be individually prescribed by a registered dietitian nutritionist and provided to participants, in order to control for the confounding effect of energy intake and diet composition. Physical activity levels will be assessed by accelerometry. Randomization will be done using the opaque, sequentially numbered envelopes technique. Participants and researchers will be blinded for continuous glucose monitoring and accelerometry data, and data will be analyzed by a blinded statistician.
Results: This study has been funded, and data collection is expected to take place between June 2024 and May 2025. Final manuscript submission should happen by August 2025.
Conclusions: The results of this project might encourage individuals living with prediabetes and T2D to engage in resistance exercise while better informing exercise specialists on how to best incorporate resistance exercise in their client's or patient's routine.
Trial registration: ClinicalTrials.gov NCT06208189; https://clinicaltrials.gov/study/NCT06208189.
International registered report identifier (irrid): PRR1-10.2196/63598.
Keywords: diabetes; glucose; insulin resistance; resistance exercise; weight training.
©Marissa Ramirez, Maja Gebauer, Christine Mermier, Jonathan Peter Little, Luotao Lin, Gabriel Palley, Yu Yu Hsiao, Roberto Ivan Mota Alvidrez, Zach A Mang, Fabiano Trigueiro Amorim, Valmor Tricoli, Flavio De Castro Magalhaes. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 05.11.2024.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


References
-
- IDF Diabetes Atlas. International Diabetes Federation. 2021. [2024-09-11]. https://diabetesatlas.org . - PubMed
-
- Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, Magliano DJ, Selvin E. Global prevalence of prediabetes. Diabetes Care. 2023;46(7):1388–1394. doi: 10.2337/dc22-2376. https://europepmc.org/abstract/MED/37196350 148937 - DOI - PMC - PubMed
-
- van Herpt TTW, Ligthart S, Leening MJG, van Hoek M, Lieverse AG, Ikram MA, Sijbrands EJG, Dehghan A, Kavousi M. Lifetime risk to progress from pre-diabetes to type 2 diabetes among women and men: comparison between American diabetes association and world health organization diagnostic criteria. BMJ Open Diabetes Res Care. 2020;8(2):e001529. doi: 10.1136/bmjdrc-2020-001529. https://drc.bmj.com/lookup/pmidlookup?view=long&pmid=33214188 8/2/e001529 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

