Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia
- PMID: 32237278
- PMCID: PMC7228321
- DOI: 10.5694/mja2.50569
Isolation and rapid sharing of the 2019 novel coronavirus (SARS-CoV-2) from the first patient diagnosed with COVID-19 in Australia
Abstract
Objectives: To describe the first isolation and sequencing of SARS-CoV-2 in Australia and rapid sharing of the isolate.
Setting: SARS-CoV-2 was isolated from a 58-year-old man from Wuhan, China who arrived in Melbourne on 19 January 2020 and was admitted to the Monash Medical Centre, Melbourne from the emergency department on 24 January 2020 with fever, cough, and progressive dyspnoea.
Major outcomes: Clinical course and laboratory features of the first reported case of COVID-19 (the illness caused by SARS-CoV-2) in Australia; isolation, whole genome sequencing, imaging, and rapid sharing of virus from the patient.
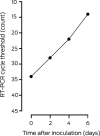
Results: A nasopharyngeal swab and sputum collected when the patient presented to hospital were each positive for SARS-CoV-2 (reverse transcription polymerase chain reaction). Inoculation of Vero/hSLAM cells with material from the nasopharyngeal swab led to the isolation of SARS-CoV-2 virus in culture. Electron microscopy of the supernatant confirmed the presence of virus particles with morphology characteristic of viruses of the family Coronaviridae. Whole genome sequencing of the viral isolate and phylogenetic analysis indicated the isolate exhibited greater than 99.99% sequence identity with other publicly available SARS-CoV-2 genomes. Within 24 hours of isolation, the first Australian SARS-CoV-2 isolate was shared with local and overseas reference laboratories and major North American and European culture collections.
Conclusions: The ability to rapidly identify, propagate, and internationally share our SARS-CoV-2 isolate is an important step in collaborative scientific efforts to deal effectively with this international public health emergency by developing better diagnostic procedures, vaccine candidates, and antiviral agents.
Keywords: Public health; Virus diseases.
© 2020 AMPCo Pty Ltd.
Conflict of interest statement
No relevant disclosures.
Figures

Comment in
-
The challenges of establishing adequate capacity for SARS-CoV-2 testing.Med J Aust. 2020 Jun;212(10):457-458. doi: 10.5694/mja2.50610. Epub 2020 May 13. Med J Aust. 2020. PMID: 32401351 Free PMC article. No abstract available.
-
Weak positive SARS-CoV-2 N2 gene results using the Xpress Xpert assay: the need for an alternate interpretative criteria in a low prevalence setting.Pathology. 2022 Feb;54(1):116-120. doi: 10.1016/j.pathol.2021.10.001. Epub 2021 Nov 23. Pathology. 2022. PMID: 34916069 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

