Clinical implementation of gene panel testing for lysosomal storage diseases
- PMID: 30548430
- PMCID: PMC6393649
- DOI: 10.1002/mgg3.527
Clinical implementation of gene panel testing for lysosomal storage diseases
Abstract
Background: The diagnostic workup in patients with a clinical suspicion of lysosomal storage diseases (LSD) is often difficult due to the variability in the clinical phenotype. The gold standard for diagnosis of LSDs consists of enzymatic testing. However, due to the sequential nature of this methodology and inconsistent genotype-phenotype correlations of certain LSDs, finding a diagnosis can be challenging.
Method: We developed and clinically implemented a gene panel covering 50 genes known to cause LSDs when mutated. Over a period of 18 months, we analyzed 150 patients who were referred for LSD testing and compared these results with the data of patients who were previously enrolled in a scheme of classical biochemical testing.
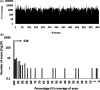
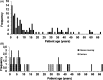
Results: Our panel was able to determine the molecular cause of the disease in 22 cases (15%), representing an increase in diagnostic yield compared to biochemical tests developed for 21 LSDs (4.6%). We were furthermore able to redirect the diagnosis of a mucolipidosis patient who was initially suspected to be affected with galactosialidosis. Several patients were identified as being affected with neuronal ceroid lipofuscinosis, which cannot readily be detected by enzyme testing. Finally, several carriers of pathogenic mutations in LSD genes related to the disease phenotype were identified as well, thus potentially increasing the diagnostic yield of the panel as heterozygous deletions cannot be detected.
Conclusion: We show that the implementation of a gene panel for LSD diagnostics results in an increased yield in comparison to classical biochemical testing. As the panel is able to cover a wider range of diseases, we propose to implement this methodology as a first-tier test in cases of an aspecific LSD presentation, while enzymatic testing remains the first choice in patients with a more distinctive clinical presentation. Positive panel results should however still be enzymatically confirmed whenever possible.
Keywords: 4MU-based enzymatic testing; diagnostic testing; gene panel sequencing; lysosomal storage disease; next-generation sequencing.
© 2018 The Authors. Molecular Genetics & Genomic Medicine published by Wiley Periodicals, Inc.
Conflict of interest statement
AG received supporting grants from Shire and Sanofi for the validation of the panel.
Figures
References
-
- Alfares, A. A. , Kelly, M. A. , McDermott, G. , Funke, B. H. , Lebo, M. S. , Baxter, S. B. , … Rehm, H. L. (2015). Results of clinical genetic testing of 2,912 probands with hypertrophic cardiomyopathy: Expanded panels offer limited additional sensitivity. Genetics in Medicine, 17(11), 880–888. 10.1038/gim.2014.205 - DOI - PubMed
-
- Anikster, Y. , Lucero, C. , Touchman, J. W. , Huizing, M. , McDowell, G. , Shotelersuk, V. , … Gahl, W. A. (1999). Identification and detection of the common 65‐kb deletion breakpoint in the nephropathic cystinosis gene (CTNS). Molecular Genetics and Metabolism, 66(2), 111–116. 10.1006/mgme.1998.2790 - DOI - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Research Materials

