Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells
- PMID: 32307869
- PMCID: PMC7294117
- DOI: 10.1111/jcmm.15277
Molecular mechanisms involved in high glucose-induced valve calcification in a 3D valve model with human valvular cells
Abstract
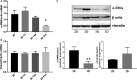
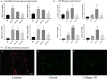
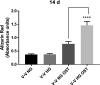
Calcific aortic valve disease (CAVD)-the most common valvular heart disease-is accelerated in diabetes and has no pharmacotherapy. Although it is known that early CAVD is associated with inflammation and osteogenesis, the molecular mechanisms involved in diabetes-associated CAVD still need to be uncovered. In this context, we have developed a 3D construct based on gelatin populated with human valvular endothelial cells (VEC) and valvular interstitial cells (VIC) and evaluated the effect of high glucose (HG) concentration on osteogenic molecules expression and on calcification mechanisms. First, we characterized the 3D model and assessed VIC remodelling properties at different time-points. Then, we exposed it to normal glucose (NG) or high glucose (HG) for 7, 14 and 21 days after which the cells were isolated, separated and investigated individually. Our results showed that encapsulated VIC actively remodel the hydrogel, as demonstrated by an increased expression of extracellular matrix (ECM) proteins and matrix metalloproteinases (MMPs). Moreover, exposure of the construct to HG triggered bone morphogenetic protein (BMP) and TGF-β signalling pathways, up-regulating expression of osteogenic molecules-BMP-2/-4, osteocalcin, osteopontin, SMADs and Runt-related transcription factor (Runx-2)-and increased calcium deposits in an osteogenic environment. These findings underline the potential of the developed 3D model as a suitable system to investigate the mechanisms of human CAVD and may help to better understand the calcification mechanisms in CAVD associated to diabetes.
Keywords: calcific aortic valve disease; high glucose levels; human valvular cells; osteogenic molecules; valve tissue engineering.
© 2020 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors confirm that there are no conflicts of interest.
Figures




References
-
- Otto CM, Prendergast B. Aortic‐valve stenosis‐from patients at risk to severe valve obstruction. N Engl J Med. 2014;21:744‐756. - PubMed
-
- Thaden JJ, Nkomo VT, Enriquez‐Sarano M. The global burden of aortic stenosis. Prog Cardiovasc Dis. 2014;56:565‐571. - PubMed
-
- Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42‐50. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Research Materials

