Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6
- PMID: 22761857
- PMCID: PMC3383673
- DOI: 10.1371/journal.pone.0039657
Satellite cells derived from obese humans with type 2 diabetes and differentiated into myocytes in vitro exhibit abnormal response to IL-6
Abstract
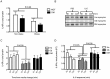
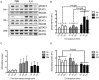
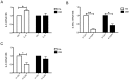
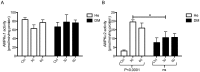
Obesity and type 2 diabetes are associated with chronically elevated systemic levels of IL-6, a pro-inflammatory cytokine with a role in skeletal muscle metabolism that signals through the IL-6 receptor (IL-6Rα). We hypothesized that skeletal muscle in obesity-associated type 2 diabetes develops a resistance to IL-6. By utilizing western blot analysis, we demonstrate that IL-6Rα protein was down regulated in skeletal muscle biopsies from obese persons with and without type 2 diabetes. To further investigate the status of IL-6 signaling in skeletal muscle in obesity-associated type 2 diabetes, we isolated satellite cells from skeletal muscle of people that were healthy (He), obese (Ob) or were obese and had type 2 diabetes (DM), and differentiated them in vitro into myocytes. Down-regulation of IL-6Rα was conserved in Ob myocytes. In addition, acute IL-6 administration for 30, 60 and 120 minutes, resulted in a down-regulation of IL-6Rα protein in Ob myocytes compared to both He myocytes (P<0.05) and DM myocytes (P<0.05). Interestingly, there was a strong time-dependent regulation of IL-6Rα protein in response to IL-6 (P<0.001) in He myocytes, not present in the other groups. Assessing downstream signaling, DM, but not Ob myocytes demonstrated a trend towards an increased protein phosphorylation of STAT3 in DM myocytes (P = 0.067) accompanied by a reduced SOCS3 protein induction (P<0.05), in response to IL-6 administration. Despite this loss of negative control, IL-6 failed to increase AMPKα2 activity and IL-6 mRNA expression in DM myocytes. There was no difference in fusion capacity of myocytes between cell groups. Our data suggest that negative control of IL-6 signaling is increased in myocytes in obesity, whereas a dysfunctional IL-6 signaling is established further downstream of IL-6Rα in DM myocytes, possibly representing a novel mechanism by which skeletal muscle function is compromised in type 2 diabetes.
Conflict of interest statement
Figures
References
-
- Richter EA, Mikines KJ, Galbo H, Kiens B. Effect of exercise on insulin action in human skeletal muscle. J Appl Physiol. 1989;66:876–885. - PubMed
-
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. - PubMed
-
- Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, et al. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

