ARID5B regulates metabolic programming in human adaptive NK cells
- PMID: 30061358
- PMCID: PMC6122973
- DOI: 10.1084/jem.20172168
ARID5B regulates metabolic programming in human adaptive NK cells
Abstract
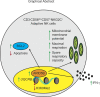
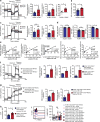
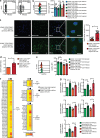
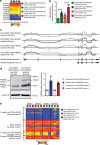
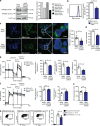
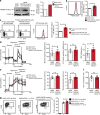
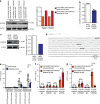
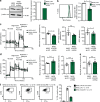
Natural killer (NK) cells with adaptive immunological properties expand and persist in response to human cytomegalovirus. Here, we explored the metabolic processes unique to these cells. Adaptive CD3-CD56dimCD57+NKG2C+ NK cells exhibited metabolic hallmarks of lymphocyte memory, including increased oxidative mitochondrial respiration, mitochondrial membrane potential, and spare respiratory capacity. Mechanistically, we found that a short isoform of the chromatin-modifying transcriptional regulator, AT-rich interaction domain 5B (ARID5B), was selectively induced through DNA hypomethylation in adaptive NK cells. Knockdown and overexpression studies demonstrated that ARID5B played a direct role in promoting mitochondrial membrane potential, expression of genes encoding electron transport chain components, oxidative metabolism, survival, and IFN-γ production. Collectively, our data demonstrate that ARID5B is a key regulator of metabolism in human adaptive NK cells, which, if targeted, may be of therapeutic value.
© 2018 Cichocki et al.
Figures

References
-
- Bantug G.R., Fischer M., Grählert J., Balmer M.L., Unterstab G., Develioglu L., Steiner R., Zhang L., Costa A.S.H., Gubser P.M., et al. . 2018. Mitochondria-Endoplasmic Reticulum Contact Sites Function as Immunometabolic Hubs that Orchestrate the Rapid Recall Response of Memory CD8+ T Cells. Immunity. 48:542–555.e6. 10.1016/j.immuni.2018.02.012 - DOI - PMC - PubMed
-
- Champagne D.P., Hatle K.M., Fortner K.A., D’Alessandro A., Thornton T.M., Yang R., Torralba D., Tomás-Cortázar J., Jun Y.W., Ahn K.H., et al. . 2016. Fine-Tuning of CD8(+) T Cell Mitochondrial Metabolism by the Respiratory Chain Repressor MCJ Dictates Protection to Influenza Virus. Immunity. 44:1299–1311. 10.1016/j.immuni.2016.02.018 - DOI - PMC - PubMed
-
- Cichocki F., Cooley S., Davis Z., DeFor T.E., Schlums H., Zhang B., Brunstein C.G., Blazar B.R., Wagner J., Diamond D.J., et al. . 2016. CD56dimCD57+NKG2C+ NK cell expansion is associated with reduced leukemia relapse after reduced intensity HCT. Leukemia. 30:456–463. 10.1038/leu.2015.260 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

