Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria
- PMID: 31004344
- PMCID: PMC6557688
- DOI: 10.1002/1873-3468.13392
Nudt8 is a novel CoA diphosphohydrolase that resides in the mitochondria
Abstract
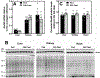
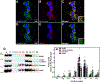
CoA regulates energy metabolism and exists in separate pools in the cytosol, peroxisomes, and mitochondria. At the whole tissue level, the concentration of CoA changes with the nutritional state by balancing synthesis and degradation; however, it is currently unclear how individual subcellular CoA pools are regulated. Liver and kidney peroxisomes contain Nudt7 and Nudt19, respectively, enzymes that catalyze CoA degradation. We report that Nudt8 is a novel CoA-degrading enzyme that resides in the mitochondria. Nudt8 has a distinctive preference for manganese ions and exhibits a broader tissue distribution than Nudt7 and Nudt19. The existence of CoA-degrading enzymes in both peroxisomes and mitochondria suggests that degradation may be a key regulatory mechanism for modulating the intracellular CoA pools.
Keywords: Coenzyme A; Nudix hydrolase; cell compartmentalization; metabolic regulation; mitochondria.
© 2019 Federation of European Biochemical Societies.
Figures

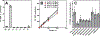

References
-
- Choudhary C, Weinert BT, Nishida Y, Verdin E, and Mann M (2014) The growing landscape of lysine acetylation links metabolism and cell signalling. Nature reviews. Molecular cell biology 15, 536–550 - PubMed
-
- Daniotti JL, Pedro MP, and Valdez Taubas J. (2017) The role of S-acylation in protein trafficking. Traffic - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

