Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial
- PMID: 31287523
- PMCID: PMC6618796
- DOI: 10.1001/jama.2019.9053
Effect of Recombinant Zoster Vaccine on Incidence of Herpes Zoster After Autologous Stem Cell Transplantation: A Randomized Clinical Trial
Erratum in
-
Incorrect Axis Scale in Figure.JAMA. 2019 Aug 27;322(8):785. doi: 10.1001/jama.2019.11467. JAMA. 2019. PMID: 31454023 Free PMC article. No abstract available.
Abstract
Importance: Herpes zoster, a frequent complication following autologous hematopoietic stem cell transplantation (HSCT), is associated with significant morbidity. A nonlive adjuvanted recombinant zoster vaccine has been developed to prevent posttransplantation zoster.
Objective: To assess the efficacy and adverse event profile of the recombinant zoster vaccine in immunocompromised autologous HSCT recipients.
Design, setting, and participants: Phase 3, randomized, observer-blinded study conducted in 167 centers in 28 countries between July 13, 2012, and February 1, 2017, among 1846 patients aged 18 years or older who had undergone recent autologous HSCT.
Interventions: Participants were randomized to receive 2 doses of either recombinant zoster vaccine (n = 922) or placebo (n = 924) administered into the deltoid muscle; the first dose was given 50 to 70 days after transplantation and the second dose 1 to 2 months thereafter.
Main outcomes and measures: The primary end point was occurrence of confirmed herpes zoster cases.
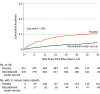
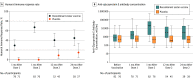
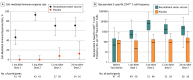
Results: Among 1846 autologous HSCT recipients (mean age, 55 years; 688 [37%] women) who received 1 vaccine or placebo dose, 1735 (94%) received a second dose and 1366 (74%) completed the study. During the 21-month median follow-up, at least 1 herpes zoster episode was confirmed in 49 vaccine and 135 placebo recipients (incidence, 30 and 94 per 1000 person-years, respectively), an incidence rate ratio (IRR) of 0.32 (95% CI, 0.22-0.44; P < .001), equivalent to 68.2% vaccine efficacy. Of 8 secondary end points, 3 showed significant reductions in incidence of postherpetic neuralgia (vaccine, n=1; placebo, n=9; IRR, 0.1; 95% CI, 0.00-0.78; P = .02) and of other prespecified herpes zoster-related complications (vaccine, n=3; placebo, n=13; IRR, 0.22; 95% CI, 0.04-0.81; P = .02) and in duration of severe worst herpes zoster-associated pain (vaccine, 892.0 days; placebo, 6275.0 days; hazard ratio, 0.62; 95% CI, 0.42-0.89; P = .01). Five secondary objectives were descriptive. Injection site reactions were recorded in 86% of vaccine and 10% of placebo recipients, of which pain was the most common, occurring in 84% of vaccine recipients (grade 3: 11%). Unsolicited and serious adverse events, potentially immune-mediated diseases, and underlying disease relapses were similar between groups at all time points.
Conclusions and relevance: Among adults who had undergone autologous HSCT, a 2-dose course of recombinant zoster vaccine compared with placebo significantly reduced the incidence of herpes zoster over a median follow-up of 21 months.
Trial registration: ClinicalTrials.gov Identifier: NCT01610414.
Conflict of interest statement
Figures
References
-
- Sahoo F, Hill JA, Xie H, et al. . Herpes zoster in autologous hematopoietic cell transplant recipients in the era of acyclovir or valacyclovir prophylaxis and novel treatment and maintenance therapies. Biol Blood Marrow Transplant. 2017;23(3):505-511. doi:10.1016/j.bbmt.2016.12.620 - DOI - PMC - PubMed

