RON is not a prognostic marker for resectable pancreatic cancer
- PMID: 22958871
- PMCID: PMC3532183
- DOI: 10.1186/1471-2407-12-395
RON is not a prognostic marker for resectable pancreatic cancer
Abstract
Background: The receptor tyrosine kinase RON exhibits increased expression during pancreatic cancer progression and promotes migration, invasion and gemcitabine resistance of pancreatic cancer cells in experimental models. However, the prognostic significance of RON expression in pancreatic cancer is unknown.
Methods: RON expression was characterized in several large cohorts, including a prospective study, totaling 492 pancreatic cancer patients and relationships with patient outcome and clinico-pathologic variables were assessed.
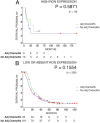
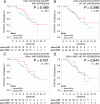
Results: RON expression was associated with outcome in a training set, but this was not recapitulated in the validation set, nor was there any association with therapeutic responsiveness in the validation set or the prospective study.
Conclusions: Although RON is implicated in pancreatic cancer progression in experimental models, and may constitute a therapeutic target, RON expression is not associated with prognosis or therapeutic responsiveness in resected pancreatic cancer.
Figures




References
-
- Neoptolemos JP, Dunn JA, Stocken DD, Almond J, Link K, Beger H, Bassi C, Falconi M, Pederzoli P, Dervenis C. et al.Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet. 2001;358(9293):1576–1585. doi: 10.1016/S0140-6736(01)06651-X. - DOI - PubMed
-
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C. et al.Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–277. doi: 10.1001/jama.297.3.267. - DOI - PubMed
-
- Samuel N, Hudson TJ. The molecular and cellular heterogeneity of pancreatic ductal adenocarcinoma. Nat Rev Gastroenterol Hepatol. 2012;9(2):77–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

