BCG Revaccination for the Prevention of Mycobacterium tuberculosis Infection
- PMID: 40334156
- PMCID: PMC12061034
- DOI: 10.1056/NEJMoa2412381
BCG Revaccination for the Prevention of Mycobacterium tuberculosis Infection
Abstract
Background: In a previous phase 2 trial, bacille Calmette-Guérin (BCG) revaccination was not shown to provide protection from primary Mycobacterium tuberculosis infection but prevented sustained M. tuberculosis infection, defined by an initial conversion on a QuantiFERON-TB (QFT) test (an interferon-γ release assay) from negative to positive, followed by two additional positive QFT tests at 3 and 6 months after the initial conversion (a secondary end point). A vaccine efficacy of 45% (95% confidence interval [CI], 6 to 68) was observed.
Methods: We performed a phase 2b, double-blind, randomized, placebo-controlled trial to evaluate the efficacy of BCG revaccination, as compared with placebo, for the prevention of sustained QFT test conversion (primary end point) in QFT test-negative, human immunodeficiency virus (HIV)-negative adolescents. Adverse events were assessed in a secondary analysis, and immunogenicity was assessed in an exploratory analysis. Vaccine efficacy was evaluated in the modified intention-to-treat population, which included all the participants who had undergone randomization, received the BCG vaccine or placebo, and had a negative QFT test 10 weeks after receipt of BCG vaccine or placebo; the last criterion was added to exclude participants with M. tuberculosis infection around the time that the vaccine or placebo was administered. Hazard ratios and 95% confidence intervals were estimated from a stratified Cox proportional-hazards model.
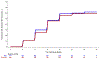
Results: A total of 1836 participants underwent randomization; 918 received the BCG vaccine, and 917 received placebo. After a median 30 months of follow-up, a sustained QFT test conversion was observed in 62 of 871 participants in the BCG-vaccine group and 59 of 849 participants in the placebo group. The hazard ratio for a sustained QFT test conversion (BCG vaccine vs. placebo) was 1.04 (95% CI, 0.73 to 1.48), for a vaccine efficacy point estimate of -3.8% (95% CI, -48.3 to 27.4). Adverse events occurred more frequently in the BCG-vaccine group than in the placebo group, and most were due to injection-site reactions (pain, redness, swelling, and ulceration). BCG revaccination induced cytokine-positive type 1 helper CD4 T cells.
Conclusions: BCG revaccination in QFT-test negative, HIV-negative adolescents did not provide protection from sustained M. tuberculosis infection. (Funded by the Gates Foundation; ClinicalTrials.gov number NCT04152161.).
Copyright © 2025 Massachusetts Medical Society.
Figures
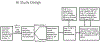
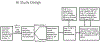



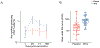
Comment in
-
BCG Revaccination to Prevent Tuberculosis.N Engl J Med. 2025 Jul 24;393(4):412. doi: 10.1056/NEJMc2507859. N Engl J Med. 2025. PMID: 40700702 No abstract available.
-
BCG Revaccination to Prevent Tuberculosis.N Engl J Med. 2025 Jul 24;393(4):412-413. doi: 10.1056/NEJMc2507859. N Engl J Med. 2025. PMID: 40700703 No abstract available.
-
BCG Revaccination to Prevent Tuberculosis. Reply.N Engl J Med. 2025 Jul 24;393(4):413. doi: 10.1056/NEJMc2507859. N Engl J Med. 2025. PMID: 40700704 No abstract available.
References
-
- Global tuberculosis report 2023. Geneva: World Health Organization; 2023. Licence: CC BY-NC-SA 3.0 IGO. Available at: https://www.who.int/publications/i/item/9789240083851.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
