The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein
- PMID: 39205158
- PMCID: PMC11360246
- DOI: 10.3390/v16081184
The Generation and Characterization of Monoclonal Antibodies against the MPXV A29L Protein
Abstract
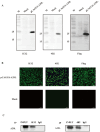
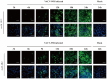
Mpox (formerly known as monkeypox) is a zoonotic disease caused by monkeypox virus (MPXV), a DNA virus belonging to the Orthopoxvirus genus, in the Poxviridae family. The disease constitutes a moderate risk to public health at the global level. The MPXV A29L protein plays a crucial role in coordinating virion assembly and facilitating important virus-host interactions. This study focused on the expression, purification, and recombinant protein synthesis of the A29L protein of MPXV using prokaryotic systems. Using hybridoma technology, we successfully generated the monoclonal antibodies (mAbs) 1E12 and 4B2, which specifically recognize the A29L protein. These mAbs were found to be suitable for use in indirect immunofluorescence assays (IFA), Western blotting, and immunoprecipitation (IP). Our investigation also revealed that mAbs 1E12 and 4B2 could detect the A27L protein, a homologous protein found in the vaccinia virus Western Reserve (VACV WR) strain, using IFA, Western blotting, and immunoprecipitation (IP). Using mAbs 1E12 and 4B2 as primary immunological probes, A27L protein expression was detected as early as 6 h postinfection with VACV WR, with increasing protein levels being observed throughout the infection. This study enhances our understanding of the protein structure and function of MPXV and contributes to the development of specific MPXV detection methods.
Keywords: A29L; VACV; monkeypox virus; monoclonal antibody.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Liu X., Jiang X., Zhu Z., Sun L., Lu H. The Novel Monkeypox Outbreak: What Should We Know and Reflect On? Zoonoses. 2022;2:1. doi: 10.15212/ZOONOSES-2022-0022. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

