Molecular dynamics simulation of the allosteric regulation of eIF4A protein from the open to closed state, induced by ATP and RNA substrates
- PMID: 24465900
- PMCID: PMC3900488
- DOI: 10.1371/journal.pone.0086104
Molecular dynamics simulation of the allosteric regulation of eIF4A protein from the open to closed state, induced by ATP and RNA substrates
Abstract
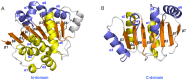
Background: Eukaryotic initiation factor 4A (eIF4A) plays a key role in the process of protein translation initiation by facilitating the melting of the 5' proximal secondary structure of eukaryotic mRNA for ribosomal subunit attachment. It was experimentally postulated that the closed conformation of the eIF4A protein bound by the ATP and RNA substrates is coupled to RNA duplex unwinding to promote protein translation initiation, rather than an open conformation in the absence of ATP and RNA substrates. However, the allosteric process of eIF4A from the open to closed state induced by the ATP and RNA substrates are not yet fully understood.
Methodology: In the present work, we constructed a series of diplex and ternary models of the eIF4A protein bound by the ATP and RNA substrates to carry out molecular dynamics simulations, free energy calculations and conformation analysis and explore the allosteric properties of eIF4A.
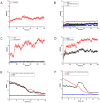
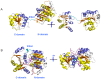
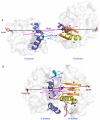
Results: The results showed that the eIF4A protein completes the conformational transition from the open to closed state via two allosteric processes of ATP binding followed by RNA and vice versa. Based on cooperative allosteric network analysis, the ATP binding to the eIF4A protein mainly caused the relative rotation of two domains, while the RNA binding caused the proximity of two domains via the migration of RNA bases in the presence of ATP. The cooperative binding of ATP and RNA for the eIF4A protein plays a key role in the allosteric transition.
Conflict of interest statement
Figures

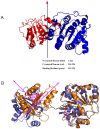


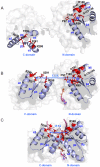


References
-
- Duncan R, Milburn SC, Hershey JW (1987) Regulated phosphorylation and low abundance of HeLa cell initiation factor eIF-4F suggest a role in translational control. Heat shock effects on eIF-4F. J Biol Chem 262: 380–388. - PubMed
-
- Spirin AS (2009) How does a scanning ribosomal particle move along the 5′-untranslated region of eukaryotic mRNA? Brownian Ratchet model. Biochemistry 48: 10688–10692. - PubMed
-
- Gingras A-C, Raught B, Sonenberg N (1999) eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu Rev Biochem 68: 913–963. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

