Contribution of TyrB26 to the Function and Stability of Insulin: STRUCTURE-ACTIVITY RELATIONSHIPS AT A CONSERVED HORMONE-RECEPTOR INTERFACE
- PMID: 27129279
- PMCID: PMC4933216
- DOI: 10.1074/jbc.M115.708347
Contribution of TyrB26 to the Function and Stability of Insulin: STRUCTURE-ACTIVITY RELATIONSHIPS AT A CONSERVED HORMONE-RECEPTOR INTERFACE
Abstract
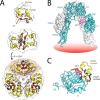
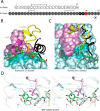
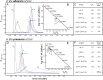
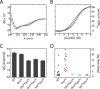
Crystallographic studies of insulin bound to receptor domains have defined the primary hormone-receptor interface. We investigated the role of Tyr(B26), a conserved aromatic residue at this interface. To probe the evolutionary basis for such conservation, we constructed 18 variants at B26. Surprisingly, non-aromatic polar or charged side chains (such as Glu, Ser, or ornithine (Orn)) conferred high activity, whereas the weakest-binding analogs contained Val, Ile, and Leu substitutions. Modeling of variant complexes suggested that the B26 side chains pack within a shallow depression at the solvent-exposed periphery of the interface. This interface would disfavor large aliphatic side chains. The analogs with highest activity exhibited reduced thermodynamic stability and heightened susceptibility to fibrillation. Perturbed self-assembly was also demonstrated in studies of the charged variants (Orn and Glu); indeed, the Glu(B26) analog exhibited aberrant aggregation in either the presence or absence of zinc ions. Thus, although Tyr(B26) is part of insulin's receptor-binding surface, our results suggest that its conservation has been enjoined by the aromatic ring's contributions to native stability and self-assembly. We envisage that such classical structural relationships reflect the implicit threat of toxic misfolding (rather than hormonal function at the receptor level) as a general evolutionary determinant of extant protein sequences.
Keywords: diabetes; hormone; non-standard mutagenesis; protein structure; receptor-tyrosine kinase.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


References
-
- Dodson G., and Steiner D. (1998) The role of assembly in insulin's biosynthesis. Curr. Opin. Struct. Biol. 8, 189–194 - PubMed
-
- Baker E. N., Blundell T. L., Cutfield J. F., Cutfield S. M., Dodson E. J., Dodson G. G., Hodgkin D. M., Hubbard R. E., Isaacs N. W., and Reynolds C. D. (1988) The structure of 2Zn pig insulin crystals at 1.5 Å resolution. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 319, 369–456 - PubMed
-
- Brange J., Ribel U., Hansen J. F., Dodson G., Hansen M. T., Havelund S., Melberg S. G., Norris F., Norris K., and Snel L. (1988) Monomeric insulins obtained by protein engineering and their medical implications. Nature 333, 679–682 - PubMed
-
- Menting J. G., Yang Y., Chan S. J., Phillips N. B., Smith B. J., Whittaker J., Wickramasinghe N. P., Whittaker L. J., Pandyarajan V., Wan Z. L., Yadav S. P., Carroll J. M., Strokes N., Roberts C. T. Jr., Ismail-Beigi F., Milewski W., Steiner D. F., Chauhan V. S., Ward C. W., Weiss M. A., and Lawrence M. C. (2014) A structural hinge in insulin enables its receptor engagement. Proc. Natl. Acad. Sci. U.S.A. 111, E3395–E3404 - PMC - PubMed
-
- Moller D. E., Yokota A., Caro J. F., and Flier J. S. (1989) Tissue-specific expression of two alternatively spliced insulin receptor mRNAs in man. Mol. Endocrinol. 3, 1263–1269 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

