Interaction of the poliovirus receptor with poliovirus
- PMID: 10618374
- PMCID: PMC26619
- DOI: 10.1073/pnas.97.1.79
Interaction of the poliovirus receptor with poliovirus
Abstract
The structure of the extracellular, three-domain poliovirus receptor (CD155) complexed with poliovirus (serotype 1) has been determined to 22-A resolution by means of cryo-electron microscopy and three-dimensional image-reconstruction techniques. Density corresponding to the receptor was isolated in a difference electron density map and fitted with known structures, homologous to those of the three individual CD155 Ig-like domains. The fit was confirmed by the location of carbohydrate moieties in the CD155 glycoprotein, the conserved properties of elbow angles in the structures of cell surface molecules with Ig-like folds, and the concordance with prior results of CD155 and poliovirus mutagenesis. CD155 binds in the poliovirus "canyon" and has a footprint similar to that of the intercellular adhesion molecule-1 receptor on human rhinoviruses. However, the orientation of the long, slender CD155 molecule relative to the poliovirus surface is quite different from the orientation of intercellular adhesion molecule-1 on rhinoviruses. In addition, the residues that provide specificity of recognition differ for the two receptors. The principal feature of receptor binding common to these two picornaviruses is the site in the canyon at which binding occurs. This site may be a trigger for initiation of the subsequent uncoating step required for viral infection.
Figures
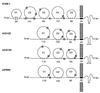



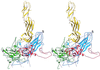
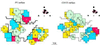
References
-
- Rueckert R R. In: Fields Virology. Fields B N, Knipe D M, Howley P M, editors. Vol. 1. Philadelphia: Lippincott; 1996. pp. 609–654.
-
- Hogle J M, Chow M, Filman D J. Science. 1985;229:1358–1365. - PubMed
-
- Rossmann M G, Arnold E, Erickson J W, Frankenberger E A, Griffith J P, Hecht H J, Johnson J E, Kamer G, Luo M, Mosser A G, et al. Nature (London) 1985;317:145–153. - PubMed
-
- Rossmann M G, Johnson J E. Annu Rev Biochem. 1989;58:533–573. - PubMed
-
- Mendelsohn C L, Wimmer E, Racaniello V R. Cell. 1989;56:855–865. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- R01 AI011219/AI/NIAID NIH HHS/United States
- R37 GM033050/GM/NIGMS NIH HHS/United States
- R01 GM056279/GM/NIGMS NIH HHS/United States
- R01 GM033050/GM/NIGMS NIH HHS/United States
- AI45976/AI/NIAID NIH HHS/United States
- R01 AI032100/AI/NIAID NIH HHS/United States
- R01 AI039485/AI/NIAID NIH HHS/United States
- R01 AI015122/AI/NIAID NIH HHS/United States
- R37 AI011219/AI/NIAID NIH HHS/United States
- P01 AI045976/AI/NIAID NIH HHS/United States
- GM33050/GM/NIGMS NIH HHS/United States
- R37 AI015122/AI/NIAID NIH HHS/United States
- AI11219/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

