NMR structure of the bovine prion protein
- PMID: 10899999
- PMCID: PMC26948
- DOI: 10.1073/pnas.97.15.8334
NMR structure of the bovine prion protein
Abstract
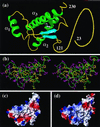
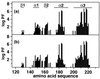
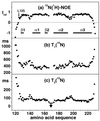
The NMR structures of the recombinant 217-residue polypeptide chain of the mature bovine prion protein, bPrP(23-230), and a C-terminal fragment, bPrP(121-230), include a globular domain extending from residue 125 to residue 227, a short flexible chain end of residues 228-230, and an N-terminal flexibly disordered "tail" comprising 108 residues for the intact protein and 4 residues for bPrP(121-230), respectively. The globular domain contains three alpha-helices comprising the residues 144-154, 173-194, and 200-226, and a short antiparallel beta-sheet comprising the residues 128-131 and 161-164. The best-defined parts of the globular domain are the central portions of the helices 2 and 3, which are linked by the only disulfide bond in bPrP. Significantly increased disorder and mobility is observed for helix 1, the loop 166-172 leading from the beta-strand 2 to helix 2, the end of helix 2 and the following loop, and the last turn of helix 3. Although there are characteristic local differences relative to the conformations of the murine and Syrian hamster prion proteins, the bPrP structure is essentially identical to that of the human prion protein. On the other hand, there are differences between bovine and human PrP in the surface distribution of electrostatic charges, which then appears to be the principal structural feature of the "healthy" PrP form that might affect the stringency of the species barrier for transmission of prion diseases between humans and cattle.
Figures


References
-
- Will R G, Ironside J W, Zeidler M, Cousens S N, Estibeiro K, Alperovitch A, Poser S, Pocchiari M, Hofman A, Smith P G. Lancet. 1996;347:921–925. - PubMed
-
- Collinge J, Palmer M S, Sidle K C L, Hill A F, Gowland I, Meads J, Asante E, Bradley R, Lawrence J D, Lantos P. Nature (London) 1995;378:779–783. - PubMed
-
- Collinge J, Sidle K C L, Meads J, Ironside J, Hill A F. Nature (London) 1996;383:685–690. - PubMed
-
- Bruce M E, Will R G, Ironside J W, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, et al. Nature (London) 1997;389:498–501. - PubMed
-
- Hill A F, Desbruslais M, Joiner S, Sidle K C L, Gowland I, Collinge J, Doey L J, Lantos P. Nature (London) 1997;389:448–450. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

