Crystal structures of the metal-dependent 2-dehydro-3-deoxy-galactarate aldolase suggest a novel reaction mechanism
- PMID: 10921867
- PMCID: PMC306599
- DOI: 10.1093/emboj/19.15.3849
Crystal structures of the metal-dependent 2-dehydro-3-deoxy-galactarate aldolase suggest a novel reaction mechanism
Abstract
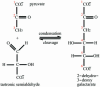
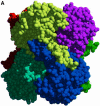
Carbon-carbon bond formation is an essential reaction in organic chemistry and the use of aldolase enzymes for the stereochemical control of such reactions is an attractive alternative to conventional chemical methods. Here we describe the crystal structures of a novel class II enzyme, 2-dehydro-3-deoxy-galactarate (DDG) aldolase from Escherichia coli, in the presence and absence of substrate. The crystal structure was determined by locating only four Se sites to obtain phases for 506 protein residues. The protomer displays a modified (alpha/beta)(8) barrel fold, in which the eighth alpha-helix points away from the beta-barrel instead of packing against it. Analysis of the DDG aldolase crystal structures suggests a novel aldolase mechanism in which a phosphate anion accepts the proton from the methyl group of pyruvate.
Figures
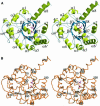





References
-
- Bacon D.J. and Anderson,W.F. (1988) A fast algorithm for rendering space-filling molecule pictures. J. Mol. Graph., 6, 219–220.
-
- Barbas C.F. III et al. (1997) Immune versus natural selection: antibody aldolases with enzymic rates but broader scope. Science, 278, 2085–2092. - PubMed
-
- Blackwell N.C. (2000) Mechanistic and structural investigation of DDG aldolase. PhD thesis, University of Leicester, Leicester, UK.
-
- Blackwell N.C., Cullis,P.M., Cooper,R.A. and Izard,T. (1999) Rhombohedral crystals of 2-dehydro-3-deoxy galactarate aldolase from Escherichia coli. Acta Crystallogr. D, 55, 1368–1369. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

