[3Fe-4S] to [4Fe-4S] cluster conversion in Desulfovibrio fructosovorans [NiFe] hydrogenase by site-directed mutagenesis
- PMID: 9751716
- PMCID: PMC21691
- DOI: 10.1073/pnas.95.20.11625
[3Fe-4S] to [4Fe-4S] cluster conversion in Desulfovibrio fructosovorans [NiFe] hydrogenase by site-directed mutagenesis
Abstract
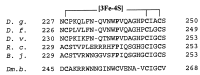
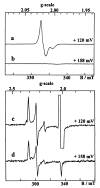
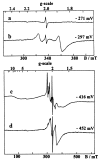
The role of the high potential [3Fe-4S]1+,0 cluster of [NiFe] hydrogenase from Desulfovibrio species located halfway between the proximal and distal low potential [4Fe-4S]2+,1+ clusters has been investigated by using site-directed mutagenesis. Proline 238 of Desulfovibrio fructosovorans [NiFe] hydrogenase, which occupies the position of a potential ligand of the lacking fourth Fe-site of the [3Fe-4S] cluster, was replaced by a cysteine residue. The properties of the mutant enzyme were investigated in terms of enzymatic activity, EPR, and redox properties of the iron-sulfur centers and crystallographic structure. We have shown on the basis of both spectroscopic and x-ray crystallographic studies that the [3Fe-4S] cluster of D. fructosovorans hydrogenase was converted into a [4Fe-4S] center in the P238 mutant. The [3Fe-4S] to [4Fe-4S] cluster conversion resulted in a lowering of approximately 300 mV of the midpoint potential of the modified cluster, whereas no significant alteration of the spectroscopic and redox properties of the two native [4Fe-4S] clusters and the NiFe center occurred. The significant decrease of the midpoint potential of the intermediate Fe-S cluster had only a slight effect on the catalytic activity of the P238C mutant as compared with the wild-type enzyme. The implications of the results for the role of the high-potential [3Fe-4S] cluster in the intramolecular electron transfer pathway are discussed.
Figures

References
-
- Adams M W W, Mortenson L E, Chen J-S. Biochim Biophys Acta. 1980;594:105–176. - PubMed
-
- Odom J M, Peck H D., Jr Annu Rev Microbiol. 1984;38:551–592. - PubMed
-
- Hatchikian E C, Fernandez V, Cammack R. In: Microbiology and Biochemistry of Strict Anaerobes Involved in Interspecies H2 Transfer. Bélaich J P, Bruschi M, Garcia J L, editors. New York: Plenum; 1990. pp. 53–73.
-
- Adams M W W. Biochim Biophys Acta. 1990;1020:115–145. - PubMed
-
- Przybyla A E, Robins J, Menon N, Peck H D. FEMS Microbiol Rev. 1992;88:109–136. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

