Three-dimensional structures of the three human class I alcohol dehydrogenases
- PMID: 11274460
- PMCID: PMC2373965
- DOI: 10.1110/ps.45001
Three-dimensional structures of the three human class I alcohol dehydrogenases
Abstract
In contrast with other animal species, humans possess three distinct genes for class I alcohol dehydrogenase and show polymorphic variation in the ADH1B and ADH1C genes. The three class I alcohol dehydrogenase isoenzymes share approximately 93% sequence identity but differ in their substrate specificity and their developmental expression. We report here the first three-dimensional structures for the ADH1A and ADH1C*2 gene products at 2.5 and 2.0 A, respectively, and the structure of the ADH1B*1 gene product in a binary complex with cofactor at 2.2 A. Not surprisingly, the overall structure of each isoenzyme is highly similar to the others. However, the substitution of Gly for Arg at position 47 in the ADH1A isoenzyme promotes a greater extent of domain closure in the ADH1A isoenzyme, whereas substitution at position 271 may account for the lower turnover rate for the ADH1C*2 isoenzyme relative to its polymorphic variant, ADH1C*1. The substrate-binding pockets of each isoenzyme possess a unique topology that dictates each isoenzyme's distinct but overlapping substrate preferences. ADH1*B1 has the most restrictive substrate-binding site near the catalytic zinc atom, whereas both ADH1A and ADH1C*2 possess amino acid substitutions that correlate with their better efficiency for the oxidation of secondary alcohols. These structures describe the nature of their individual substrate-binding pockets and will improve our understanding of how the metabolism of beverage ethanol affects the normal metabolic processes performed by these isoenzymes.
Figures

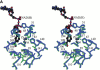
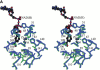


References
-
- Bacon, D.J. and Anderson, W.F. 1988. A fast algorithm for rendering space-filling molecule pictures. J. Molec. Graph. 6 219–220.
-
- Bosron, W.F. and Li, T.-K. 1987. Catalytic properties of human liver alcohol dehydrogenase isoenzymes. Enzyme 37 19–28. - PubMed
-
- Bosron, W.F., Magnes, L.J., and Li, T.-K. 1983. Kinetic and electrophoretic properties of native and recombined isoenzymes of human liver alcohol dehydrogenase. Biochemistry 22 1852–1857. - PubMed
-
- Brünger, A.T. and Rice, L.M. 1997. Crystallographic refinement by simulated annealing: Methods and applications. Methods Enzymol. 277 243–269. - PubMed
-
- Burnell, J.C., Carr, L.G., Dwulet, F.E., Edenberg, H.J., Li, T.-K., and Bosron, W.F. 1987. The human β3 alcohol dehydrogenase subunit differs from β1 by a cys for arg-369 substitution which decreases NAD(H) binding. Biochem. Biophys. Res. Commun. 146 1227–1233. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

