Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes
- PMID: 12538866
- PMCID: PMC298693
- DOI: 10.1073/pnas.0231020100
Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes
Abstract
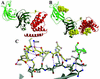
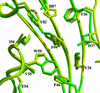
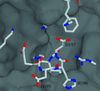
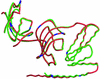
The ability to bind immunosuppressive drugs such as cyclosporin and FK506 defines the immunophilin family of proteins, and the FK506-binding proteins form the FKBP subfamily of immunophilins. Some FKBPs, notably FKBP12 (the 12-kDa FK506-binding protein), have defined roles in regulating ion channels or cell signaling, and well established structures. Other FKBPs, especially the larger ones, participate in important biological processes, but their exact roles and the structural bases for these roles are poorly defined. FKBP51 (the 51-kDa FKBP) associates with heat shock protein 90 (Hsp90) and appears in functionally mature steroid receptor complexes. In New World monkeys, FKBP51 has been implicated in cortisol resistance. We report here the x-ray structures of human FKBP51, to 2.7 A, and squirrel monkey FKBP51, to 2.8 A, by using multiwavelength anomalous dispersion phasing. FKBP51 is composed of three domains: two consecutive FKBP domains and a three-unit repeat of the TPR (tetratricopeptide repeat) domain. This structure of a multi-FKBP domain protein clarifies the arrangement of these domains and their possible interactions with other proteins. The two FKBP domains differ by an insertion in the second that affects the formation of the progesterone receptor complex.
Figures


References
-
- Siekierka J J, Hung S H Y, Poe M, Lin C S, Sigal N H. Nature. 1989;341:755–757. - PubMed
-
- Harding M W, Galat A, Uehling D E, Schreiber S L. Nature. 1989;341:758–760. - PubMed
-
- Van Duyne G D, Standaert R F, Karplus P A, Schreiber S L, Clardy J. Science. 1991;252:839–842. - PubMed
-
- Liu J. Cell. 1991;66:807–815. - PubMed
-
- O'Keefe S J, Tamura J, Kincaid R F, Tocci M J, O'Neill M A. Nature. 1992;357:692–694. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

