Histidine 114 Is Critical for ATP Hydrolysis by the Universally Conserved ATPase YchF
- PMID: 26018081
- PMCID: PMC4513122
- DOI: 10.1074/jbc.M114.598227
Histidine 114 Is Critical for ATP Hydrolysis by the Universally Conserved ATPase YchF
Abstract
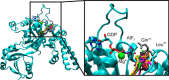
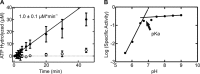
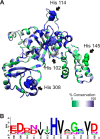
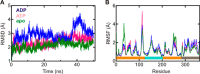
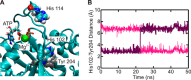
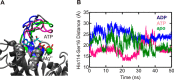
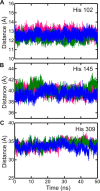
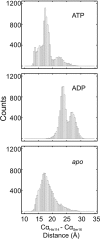
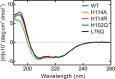
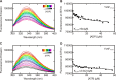
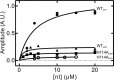
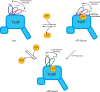
GTPases perform a wide range of functions, ranging from protein synthesis to cell signaling. Of all known GTPases, only eight are conserved across all three domains of life. YchF is one of these eight universally conserved GTPases; however, its cellular function and enzymatic properties are poorly understood. YchF differs from the classical GTPases in that it has a higher affinity for ATP than for GTP and is a functional ATPase. As a hydrophobic amino acid-substituted ATPase, YchF does not possess the canonical catalytic Gln required for nucleotide hydrolysis. To elucidate the catalytic mechanism of ATP hydrolysis by YchF, we have taken a two-pronged approach combining classical biochemical and in silico techniques. The use of molecular dynamics simulations allowed us to complement our biochemical findings with information about the structural dynamics of YchF. We have thereby identified the highly conserved His-114 as critical for the ATPase activity of YchF from Escherichia coli. His-114 is located in a flexible loop of the G-domain, which undergoes nucleotide-dependent conformational changes. The use of a catalytic His is also observed in the hydrophobic amino acid-substituted GTPase RbgA and is an identifier of the translational GTPase family.
Keywords: ATPase; HAS-ATPase; YchF; catalytic mechanism; conformational change; molecular dynamics; molecular switch; nucleotide binding; pre-steady-state kinetics; ribosome.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Bourne H. R., Sanders D. A., McCormick F. (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348, 125–132 - PubMed
-
- Caldon C. E., March P. E. (2003) Function of the universally conserved bacterial GTPases. Curr. Opin. Microbiol 6, 135–139 - PubMed
-
- Bourne H. R., Sanders D. A., McCormick F. (1991) The GTPase superfamily: conserved structure and molecular mechanism. Nature 349, 117–127 - PubMed
-
- Sprang S. R. (1997) G protein mechanisms: insights from structural analysis. Annu. Rev. Biochem. 66, 639–678 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

