Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide
- PMID: 15861141
- PMCID: PMC1142576
- DOI: 10.1038/sj.emboj.7600635
Crystal structure of the human urokinase plasminogen activator receptor bound to an antagonist peptide
Abstract
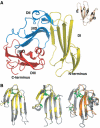
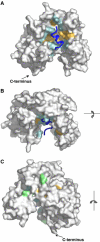
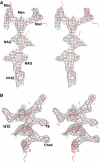
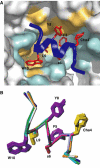
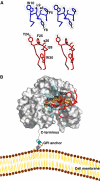
We report the crystal structure of a soluble form of human urokinase-type plasminogen activator receptor (uPAR/CD87), which is expressed at the invasive areas of the tumor-stromal microenvironment in many human cancers. The structure was solved at 2.7 A in association with a competitive peptide inhibitor of the urokinase-type plasminogen activator (uPA)-uPAR interaction. uPAR is composed of three consecutive three-finger domains organized in an almost circular manner, which generates both a deep internal cavity where the peptide binds in a helical conformation, and a large external surface. This knowledge combined with the discovery of a convergent binding motif shared by the antagonist peptide and uPA allowed us to build a model of the human uPA-uPAR complex. This model reveals that the receptor-binding module of uPA engages the uPAR central cavity, thus leaving the external receptor surface accessible for other protein interactions (vitronectin and integrins). By this unique structural assembly, uPAR can orchestrate the fine interplay with the partners that are required to guide uPA-focalized proteolysis on the cell surface and control cell adhesion and migration.
Figures

References
-
- Appella E, Robinson EA, Ullrich SJ, Stoppelli MP, Corti A, Cassani G, Blasi F (1987) The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem 262: 4437–4440 - PubMed
-
- Bdeir K, Kuo A, Mazar A, Sachais BS, Xiao W, Gawlak S, Harris S, Higazi AA, Cines DB (2000) A region in domain II of the urokinase receptor required for urokinase binding. J Biol Chem 275: 28532–28538 - PubMed
-
- Blasi F, Carmeliet P (2002) uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 3: 932–943 - PubMed
-
- Brooks BR, Karplus M, Petit BM (1988) Proteins: A Theoretical Perspective of Dynamics, Structure and Thermodynamics. New York: Wiley
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

