Structural basis of family-wide Rab GTPase recognition by rabenosyn-5
- PMID: 16034420
- PMCID: PMC1360218
- DOI: 10.1038/nature03798
Structural basis of family-wide Rab GTPase recognition by rabenosyn-5
Abstract
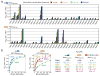
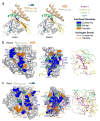
Rab GTPases regulate all stages of membrane trafficking, including vesicle budding, cargo sorting, transport, tethering and fusion. In the inactive (GDP-bound) conformation, accessory factors facilitate the targeting of Rab GTPases to intracellular compartments. After nucleotide exchange to the active (GTP-bound) conformation, Rab GTPases interact with functionally diverse effectors including lipid kinases, motor proteins and tethering complexes. How effectors distinguish between homologous Rab GTPases represents an unresolved problem with respect to the specificity of vesicular trafficking. Using a structural proteomic approach, we have determined the specificity and structural basis underlying the interaction of the multivalent effector rabenosyn-5 with the Rab family. The results demonstrate that even the structurally similar effector domains in rabenosyn-5 can achieve highly selective recognition of distinct subsets of Rab GTPases exclusively through interactions with the switch and interswitch regions. The observed specificity is determined at a family-wide level by structural diversity in the active conformation, which governs the spatial disposition of critical conserved recognition determinants, and by a small number of both positive and negative sequence determinants that allow further discrimination between Rab GTPases with similar switch conformations.
Conflict of interest statement
Figures


References
-
- Pfeffer SR. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 2001;11:487–491. - PubMed
-
- Zerial M, McBride H. Rab proteins as membrane organizers. Nat Rev Mol Cell Biol. 2001;2:107–117. - PubMed
-
- Sivars U, Aivazian D, Pfeffer SR. Yip3 catalyses the dissociation of endosomal Rab-GDI complexes. Nature. 2003;425:856–859. - PubMed
-
- Rak A, et al. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 2003;302:646–650. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

