Crystal structure of methylenetetrahydromethanopterin reductase (Mer) in complex with coenzyme F420: Architecture of the F420/FMN binding site of enzymes within the nonprolyl cis-peptide containing bacterial luciferase family
- PMID: 15937276
- PMCID: PMC2253363
- DOI: 10.1110/ps.041289805
Crystal structure of methylenetetrahydromethanopterin reductase (Mer) in complex with coenzyme F420: Architecture of the F420/FMN binding site of enzymes within the nonprolyl cis-peptide containing bacterial luciferase family
Abstract
Methylenetetratetrahydromethanopterin reductase (Mer) is involved in CO(2) reduction to methane in methanogenic archaea and catalyses the reversible reduction of methylenetetrahydromethanopterin (methylene-H(4)MPT) to methyl-H(4)MPT with coenzyme F(420)H(2), which is a reduced 5'-deazaflavin. Mer was recently established as a TIM barrel structure containing a nonprolyl cis-peptide bond but the binding site of the substrates remained elusive. We report here on the crystal structure of Mer in complex with F(420) at 2.6 A resolution. The isoalloxazine ring is present in a pronounced butterfly conformation, being induced from the Re-face of F(420) by a bulge that contains the non-prolyl cis-peptide bond. The bindingmode of F(420) is very similar to that in F(420)-dependent alcohol dehydrogenase Adf despite the low sequence identity of 21%. Moreover, binding of F(420) to the apoenzyme was only associated with minor conformational changes of the polypeptide chain. These findings allowed us to build an improved model of FMN into its binding site in bacterial luciferase, which belongs to the same structural family as Mer and Adf and also contains a nonprolyl cis-peptide bond in an equivalent position.
Figures

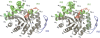

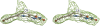
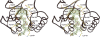
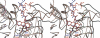

References
-
- Abu-Soud, H.M., Clark, A.C., Francisco, W.A., Baldwin, T.O., and Raushel, F.M. 1993. Kinetic destabilization of the hydroperoxy flavin intermediate by site-directed modification of the reactive thiol in bacterial luciferase. J. Biol. Chem. 268 7699–7706. - PubMed
-
- Acharya, P., Goenrich, M., Hagemeier, C.H., Demmer, U., Vorholt, J., Thauer, R.K., and Ermler, U. 2005. How an enzyme binds the C1-carrier tetrahydromethanopterin: Structure of the tetrahydromethanopterin dependent formaldehyde-activating enzyme Fae from Methylobacterium extorquens AM1. J. Biol. Chem. (in press). - PubMed
-
- Ahn, H.J., Yoon, H.J., Lee 2d, B., and Suh, S.W. 2004. Crystal structure of chorismate synthase: A novel FMN-binding protein fold and functional insights. J. Mol. Biol. 336 903–915. - PubMed
-
- Aufhammer, S.W., Warkentin, E., Berk, H., Shima, S., Thauer, R.K., and Ermler, U. 2004. Coenzyme binding in F420-dependent secondary alcohol dehydrogenase, a member of the bacterial luciferase family. Structure 12 361–370. - PubMed
-
- Bacon, D.J. and Anderson, W.F. 1988. A fast algorithm for rendering space-filling molecule pictures. J. Mol. Graph. 6 219–220.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

