Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
- PMID: 17535895
- PMCID: PMC1877987
- DOI: 10.1073/pnas.0702254104
Bisphosphonates target multiple sites in both cis- and trans-prenyltransferases
Abstract
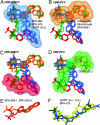
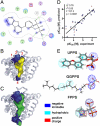
Bisphosphonate drugs (e.g., Fosamax and Zometa) are thought to act primarily by inhibiting farnesyl diphosphate synthase (FPPS), resulting in decreased prenylation of small GTPases. Here, we show that some bisphosphonates can also inhibit geranylgeranyl diphosphate synthase (GGPPS), as well as undecaprenyl diphosphate synthase (UPPS), a cis-prenyltransferase of interest as a target for antibacterial therapy. Our results on GGPPS (10 structures) show that there are three bisphosphonate-binding sites, consisting of FPP or isopentenyl diphosphate substrate-binding sites together with a GGPP product- or inhibitor-binding site. In UPPS, there are a total of four binding sites (in five structures). These results are of general interest because they provide the first structures of GGPPS- and UPPS-inhibitor complexes, potentially important drug targets, in addition to revealing a remarkably broad spectrum of binding modes not seen in FPPS inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
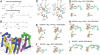



References
-
- Russell RG. Ann NY Acad Sci. 2006;1068:367–401. - PubMed
-
- Martin MB, Grimley JS, Lewis JC, Heath HT, III, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, et al. J Med Chem. 2001;44:909–916. - PubMed
-
- Kavanagh KL, Dunford JE, Bunkoczi G, Russell RG, Oppermann U. J Biol Chem. 2006;281:22004–22012. - PubMed
-
- Li H, Huang J, Jiang X, Seefeld M, McQueney M, Macarron R. J Biomol Screen. 2003;8:712–715. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Molecular Biology Databases

