Structural insights into fibronectin type III domain-mediated signaling
- PMID: 17261313
- PMCID: PMC2583400
- DOI: 10.1016/j.jmb.2006.10.017
Structural insights into fibronectin type III domain-mediated signaling
Abstract
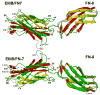
The alternatively spliced type III extradomain B (EIIIB) of fibronectin (FN) is expressed only during embryogenesis, wound healing and tumorigenesis. The biological function of this domain is unclear. We describe here the first crystal structure of the interface between alternatively spliced EIIIB and its adjacent FN type III domain 8 (FN B-8). The opened CC' loop of EIIIB, and the rotation and tilt of EIIIB allow good access to the FG loop of FN-8, which is normally hindered by the CC' loop of FN-7. In addition, the AGEGIP sequence of the CC'' loop of EIIIB replaces the NGQQGN sequence of the CC' loop of FN-7. Finally, the CC'' loop of EIIIB forms an acidic groove with FN-8. These structural findings warrant future studies directed at identifying potential binding partners for FN B-8 interface, linking EIIIB to skeletal and cartilaginous development, wound healing, and tumorigenesis, respectively.
Figures





References
-
- Hynes RO. Fibronectins. New York: Springer Verlag; 1990.
-
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development (Camb) 1993;119:1079–1091. - PubMed
-
- Leahy DJ, Aukhil I, Erickson HP. 2.0 Å crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell. 1996;84:155–164. - PubMed
-
- Pierschbacher MD, Ruoslahti E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

