Calcium-induced folding of a fragment of calmodulin composed of EF-hands 2 and 3
- PMID: 17473011
- PMCID: PMC2206659
- DOI: 10.1110/ps.072777107
Calcium-induced folding of a fragment of calmodulin composed of EF-hands 2 and 3
Abstract
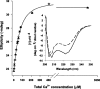
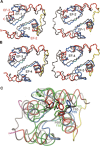
Calmodulin (CaM) is an EF-hand protein composed of two calcium (Ca(2+))-binding EF-hand motifs in its N-domain (EF-1 and EF-2) and two in its C-domain (EF-3 and EF-4). In this study, we examined the structure, dynamics, and Ca(2+)-binding properties of a fragment of CaM containing only EF-2 and EF-3 and the intervening linker sequence (CaM2/3). Based on NMR spectroscopic analyses, Ca(2+)-free CaM2/3 is predominantly unfolded, but upon binding Ca(2+), adopts a monomeric structure composed of two EF-hand motifs bridged by a short antiparallel beta-sheet. Despite having an "even-odd" pairing of EF-hands, the tertiary structure of CaM2/3 is similar to both the "odd-even" paired N- and C-domains of Ca(2+)-ligated CaM, with the conformationally flexible linker sequence adopting the role of an inter-EF-hand loop. However, unlike either CaM domain, CaM2/3 exhibits stepwise Ca(2+) binding with a K (d1) = 30 +/- 5 microM to EF-3, and a K (d2) > 1000 microM to EF-2. Binding of the first equivalent of Ca(2+) induces the cooperative folding of CaM2/3. In the case of native CaM, stacking interactions between four conserved aromatic residues help to hold the first and fourth helices of each EF-hand domain together, while the loop between EF-hands covalently tethers the second and third helices. In contrast, these aromatic residues lie along the second and third helices of CaM2/3, and thus are positioned adjacent to the loop between its "even-odd" paired EF-hands. This nonnative hydrophobic core packing may contribute to the weak Ca(2+) affinity exhibited by EF-2 in the context of CaM2/3.
Figures





References
-
- Andre I. and Linse, S. 2002. Measurement of Ca2+-binding constants of proteins and presentation of the CaLigator software. Anal. Biochem. 305: 195–205. - PubMed
-
- Babu Y.S., Bugg, C.E., and Cook, W.J. 1988. Structure of calmodulin refined at 2.2 Å resolution. J. Mol. Biol. 204: 191–204. - PubMed
-
- Biekofsky R.R., Martin, S.R., Browne, J.P., Bayley, P.M., and Feeney, J. 1998. Ca2+ coordination to backbone carbonyl oxygen atoms in calmodulin and other EF-hand proteins: 15N chemical shifts as probes for monitoring individual-site Ca2+ coordination. Biochemistry 37: 7617–7629. - PubMed
-
- Brent R. 1998. Protein expression. In Current protocols in molecular biology (eds. F. Ausubel et al.), pp. 16.10.11–16.21.19. John Wiley & Sons, New York.
-
- Brunger A.T., Adams, P.D., Clore, G.M., DeLano, W.L., Gros, P., Grosse-Kunstleve, R.W., Jiang, J.S., Kuszewski, J., Nilges, M., Pannu, N.S., et al. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr. D Biol. Crystallogr. 54: 905–921. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

