Structural basis for intramembrane proteolysis by rhomboid serine proteases
- PMID: 17190827
- PMCID: PMC1766407
- DOI: 10.1073/pnas.0609773104
Structural basis for intramembrane proteolysis by rhomboid serine proteases
Abstract
Intramembrane proteases catalyze peptide bond cleavage of integral membrane protein substrates. This activity is crucial for many biological and pathological processes. Rhomboids are evolutionarily widespread intramembrane serine proteases. Here, we present the 2.3-A-resolution crystal structure of a rhomboid from Escherichia coli. The enzyme has six transmembrane helices, five of which surround a short TM4, which starts deep within the membrane at the catalytic serine residue. Thus, the catalytic serine is in an externally exposed cavity, which provides a hydrophilic environment for proteolysis. Our results reveal a mechanism to enable water-dependent catalysis at the depth of the hydrophobic milieu of the membrane and suggest how substrates gain access to the sequestered rhomboid active site.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

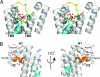
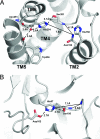

Comment in
-
Membrane-embedded protease poses for photoshoot.Proc Natl Acad Sci U S A. 2007 Jan 9;104(2):401-2. doi: 10.1073/pnas.0610236103. Epub 2007 Jan 3. Proc Natl Acad Sci U S A. 2007. PMID: 17213330 Free PMC article. No abstract available.
References
-
- Brown MS, Ye J, Rawson RB, Goldstein JL. Cell. 2000;100:391–398. - PubMed
-
- Wolfe MS, Kopan R. Science. 2004;305:1119–1123. - PubMed
-
- Marjaux E, Hartmann D, De Strooper B. Neuron. 2004;42:189–192. - PubMed
-
- Selkoe D, Kopan R. Annu Rev Neurosci. 2003;26:565–597. - PubMed
-
- Rawson RB, Zelenski NG, Nijhawan D, Ye J, Sakai J, Hasan MT, Chang TY, Brown MS, Goldstein JL. Mol Cell. 1997;1:47–57. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

