Molecular architecture of strictosidine glucosidase: the gateway to the biosynthesis of the monoterpenoid indole alkaloid family
- PMID: 17890378
- PMCID: PMC2048697
- DOI: 10.1105/tpc.106.045682
Molecular architecture of strictosidine glucosidase: the gateway to the biosynthesis of the monoterpenoid indole alkaloid family
Abstract
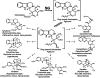
Strictosidine beta-D-glucosidase (SG) follows strictosidine synthase (STR1) in the production of the reactive intermediate required for the formation of the large family of monoterpenoid indole alkaloids in plants. This family is composed of approximately 2000 structurally diverse compounds. SG plays an important role in the plant cell by activating the glucoside strictosidine and allowing it to enter the multiple indole alkaloid pathways. Here, we report detailed three-dimensional information describing both native SG and the complex of its inactive mutant Glu207Gln with the substrate strictosidine, thus providing a structural characterization of substrate binding and identifying the amino acids that occupy the active site surface of the enzyme. Structural analysis and site-directed mutagenesis experiments demonstrate the essential role of Glu-207, Glu-416, His-161, and Trp-388 in catalysis. Comparison of the catalytic pocket of SG with that of other plant glucosidases demonstrates the structural importance of Trp-388. Compared with all other glucosidases of plant, bacterial, and archaeal origin, SG's residue Trp-388 is present in a unique structural conformation that is specific to the SG enzyme. In addition to STR1 and vinorine synthase, SG represents the third structural example of enzymes participating in the biosynthetic pathway of the Rauvolfia alkaloid ajmaline. The data presented here will contribute to deciphering the structure and reaction mechanism of other higher plant glucosidases.
Figures




References
-
- Barleben, L., Ma, X.Y., Koepke, J., Peng, G., Michel, H., and Stöckigt, J. (2005). Expression, purification, crystallization and preliminary X-ray analysis of strictosidine glucosidase, an enzyme initiating biosynthetic pathways to a unique diversity of indole alkaloid skeletons. Biochim. Biophys. Acta 1747 89–92. - PubMed
-
- Barrett, T., Suresh, C.G., Tolley, S.P., Dodson, E.J., and Hughes, M.A. (1995). The crystal structure of a cyanogenic beta-glucosidase from white clover, a family 1 glycosyl hydrolase. Structure 3 951–960. - PubMed
-
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. - PubMed
-
- Brünger, A.T. (1993). Assessment of phase accuracy by cross validation: The free R value. Methods and applications. Acta Crystallogr. D Biol. Crystallogr. 49 24–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

