Long range dynamic effects of point-mutations trap a response regulator in an active conformation
- PMID: 20828564
- PMCID: PMC2949504
- DOI: 10.1016/j.febslet.2010.08.051
Long range dynamic effects of point-mutations trap a response regulator in an active conformation
Abstract
When a point-mutation in a protein elicits a functional change, it is most common to assign this change to local structural perturbations. Here we show that point-mutations, distant from an essential highly dynamic kinase recognition loop in the response regulator Spo0F, lock this loop in an active conformation. This 'conformational trapping' results in functionally hyperactive Spo0F. Consequently, point-mutations are seen to affect functionally critical motions both close to and far from the mutational site.
Copyright © 2010 Federation of European Biochemical Societies. Published by Elsevier B.V. All rights reserved.
Figures

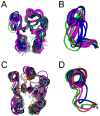

 ” Spo0F; red “
” Spo0F; red “ ” Spo0F-BeF3; orange “
” Spo0F-BeF3; orange “ ” L66A; purple “
” L66A; purple “ ” I90A; black “*” H101A.
” I90A; black “*” H101A.References
-
- Hoch JA, Silhavy TJ. Two-component signal transduction. ASM Press; Washington, D.C: 1995.
-
- Hoch JA. Two-component and phosphorelay signal transduction. Curr Opin Microbiol. 2000;3:165–70. - PubMed
-
- Sharp JS, Sullivan DM, Cavanagh J, Tomer KB. Measurement of multisite oxidation kinetics reveals an active site conformational change in Spo0F as a result of protein oxidation. Biochemistry. 2006;45:6260–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

