Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities
- PMID: 18359860
- PMCID: PMC2271164
- DOI: 10.1110/ps.073295308
Structure dissection of human progranulin identifies well-folded granulin/epithelin modules with unique functional activities
Abstract
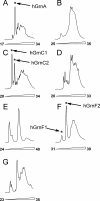
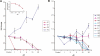
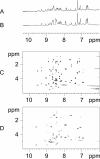
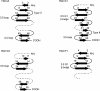
Progranulin is a secreted protein with important functions in several physiological and pathological processes, such as embryonic development, host defense, and wound repair. Autosomal dominant mutations in the progranulin gene cause frontotemporal dementia, while overexpression of progranulin promotes the invasive progression of a range of tumors, including those of the breast and the brain. Structurally, progranulin consists of seven-and-a-half tandem repeats of the granulin/epithelin module (GEM), several of which have been isolated as discrete 6-kDa GEM peptides. We have expressed all seven human GEMs using recombinant DNA in Escherichia coli. High-resolution NMR showed that only the three GEMs, hGrnA, hGrnC, and hGrnF, contain relatively well-defined three-dimensional structures in solution, while others are mainly mixtures of poorly structured disulfide isomers. The three-dimensional structures of hGrnA, hGrnC, and hGrnF contain a stable stack of two beta-hairpins in their N-terminal subdomains, but showed a more flexible C-terminal subdomain. Interestingly, of the well-structured GEMs, hGrnA demonstrated potent growth inhibition of a breast cancer cell line, while hGrnF was stimulatory. Poorly folded peptides were either weakly inhibitory or without activity. The functionally active and structurally well-characterized human hGrnA offers a unique opportunity for detailed structure-function studies of these important GEM proteins as novel members of mammalian growth factors.
Figures



References
-
- Baba, T., Hoff H.B., III, Nemoto, H., Lee, H., Orth, J., Arai, Y., Gerton, G.L. Acrogranin, an acrosomal cysteine-rich glycoprotein, is the precursor of the growth-modulating peptides, granulins, and epithelins, and is expressed in somatic as well as male germ cells. Mol. Reprod. Dev. 1993;34:233–243. - PubMed
-
- Baker, M., Mackenzie, I.R., Pickering-Brown, S.M., Gass, J., Rademakers, R., Lindholm, C., Snowden, J., Adamson, J., Sadovnick, A.D., Rollinson, S., et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. - PubMed
-
- Bateman, A., Bennett, H.P. Granulins: The structure and function of an emerging family of growth factors. J. Endocrinol. 1998;158:145–151. - PubMed
-
- Bateman, A., Belcourt, D., Bennett, H., Lazure, C., Solomon, S. Granulins, a novel class of peptide from leukocytes. Biochem. Biophys. Res. Commun. 1990;173:1161–1168. - PubMed
-
- Belcourt, D.R., Lazure, C., Bennett, H.P. Isolation and primary structure of the three major forms of granulinlike peptides from hematopoietic tissues of a teleost fish (Cyprinus carpio) J. Biol. Chem. 1993;268:9230–9237. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

