Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300
- PMID: 26851278
- PMCID: PMC4807259
- DOI: 10.1074/jbc.R115.692020
Role of Intrinsic Protein Disorder in the Function and Interactions of the Transcriptional Coactivators CREB-binding Protein (CBP) and p300
Abstract
The transcriptional coactivators CREB-binding protein (CBP) and p300 undergo a particularly rich set of interactions with disordered and partly ordered partners, as a part of their ubiquitous role in facilitating transcription of genes. CBP and p300 contain a number of small structured domains that provide scaffolds for the interaction of disordered transactivation domains from a wide variety of partners, including p53, hypoxia-inducible factor 1α (HIF-1α), NF-κB, and STAT proteins, and are the targets for the interactions of disordered viral proteins that compete with cellular factors to disrupt signaling and subvert the cell cycle. The functional diversity of the CBP/p300 interactome provides an excellent example of the power of intrinsic disorder to facilitate the complexity of living systems.
Keywords: IDP; IDR; STAT transcription factor; cAMP response element-binding protein (CREB); coupled folding and binding; hypoxia-inducible factor (HIF); intrinsically disordered protein; intrinsically disordered region; protein-protein interaction; structure-function; transcriptional activation; transcriptional coactivator; viral oncoprotein.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


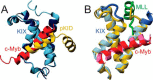
References
-
- Minezaki Y., Homma K., Kinjo A. R., and Nishikawa K. (2006) Human transcription factors contain a high fraction of intrinsically disordered regions essential for transcriptional regulation. J. Mol. Biol. 359, 1137–1149 - PubMed
-
- Sigler P. B. (1988) Transcriptional activation: acid blobs and negative noodles. Nature 333, 210–212 - PubMed
-
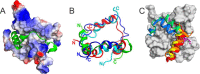
- Radhakrishnan I., Pérez-Alvarado G. C., Parker D., Dyson H. J., Montminy M. R., and Wright P. E. (1997) Solution structure of the KIX domain of CBP bound to the transactivation domain of CREB: a model for activator:coactivator interactions. Cell 91, 741–752 - PubMed
-
- Wright P. E., and Dyson H. J. (1999) Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J. Mol. Biol. 293, 321–331 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

