Structure, function, and targets of the transcriptional regulator SvtR from the hyperthermophilic archaeal virus SIRV1
- PMID: 19535331
- PMCID: PMC2755947
- DOI: 10.1074/jbc.M109.029850
Structure, function, and targets of the transcriptional regulator SvtR from the hyperthermophilic archaeal virus SIRV1
Abstract
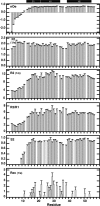
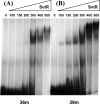
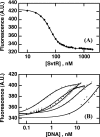
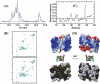
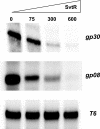
We have characterized the structure and the function of the 6.6-kDa protein SvtR (formerly called gp08) from the rod-shaped virus SIRV1, which infects the hyperthermophilic archaeon Sulfolobus islandicus that thrives at 85 degrees C in hot acidic springs. The protein forms a dimer in solution. The NMR solution structure of the protein consists of a ribbon-helix-helix (RHH) fold between residues 13 and 56 and a disordered N-terminal region (residues 1-12). The structure is very similar to that of bacterial RHH proteins despite the low sequence similarity. We demonstrated that the protein binds DNA and uses its beta-sheet face for the interaction like bacterial RHH proteins. To detect all the binding sites on the 32.3-kb SIRV1 linear genome, we designed and performed a global genome-wide search of targets based on a simplified electrophoretic mobility shift assay. Four targets were recognized by the protein. The strongest binding was observed with the promoter of the gene coding for a virion structural protein. When assayed in a host reconstituted in vitro transcription system, the protein SvtR (Sulfolobus virus transcription regulator) repressed transcription from the latter promoter, as well as from the promoter of its own gene.
Figures
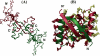


References
-
- Torsvik T., Dundas I. D. (1974) Nature 248, 680–681 - PubMed
-
- Prangishvili D., Stedman K., Zillig W. (2001) Trends Microbiol. 9, 39–43 - PubMed
-
- Prangishvili D., Garrett R. A. (2004) Biochem. Soc. Trans. 32, 204–208 - PubMed
-
- Prangishvili D., Forterre P., Garrett R. A. (2006) Nat. Rev. Microbiol. 4, 837–848 - PubMed
-
- Prangishvili D., Garrett R. A., Koonin E. V. (2006) Virus Res. 117, 52–67 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

