α-helical structures drive early stages of self-assembly of amyloidogenic amyloid polypeptide aggregate formation in membranes
- PMID: 24071712
- PMCID: PMC3784961
- DOI: 10.1038/srep02781
α-helical structures drive early stages of self-assembly of amyloidogenic amyloid polypeptide aggregate formation in membranes
Abstract
The human islet amyloid polypeptide (hIAPP) is the primary component in the toxic islet amyloid deposits in type-2 diabetes. hIAPP self-assembles to aggregates that permeabilize membranes and constitutes amyloid plaques. Uncovering the mechanisms of amyloid self-assembly is the key to understanding amyloid toxicity and treatment. Although structurally similar, hIAPP's rat counterpart, the rat islet amyloid polypeptide (rIAPP), is non-toxic. It has been a puzzle why these peptides behave so differently. We combined multiscale modelling and theory to explain the drastically different dynamics of hIAPP and rIAPP: The differences stem from electrostatic dipolar interactions. hIAPP forms pentameric aggregates with the hydrophobic residues facing the membrane core and stabilizing water-conducting pores. We give predictions for pore sizes, the number of hIAPP peptides, and aggregate morphology. We show the importance of curvature-induced stress at the early stages of hIAPP assembly and the α-helical structures over β-sheets. This agrees with recent fluorescence spectroscopy experiments.
Figures
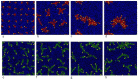
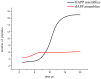

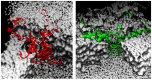

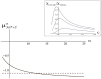
 . From the top to the bottom:
. From the top to the bottom:  = 1, 2, 3.
= 1, 2, 3.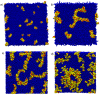

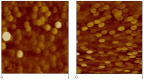
References
-
- Merlini G. & Bellotti V. Molecular mechanisms of amyloidosis. N. Engl. J. Med. 349, 583–596 (2003). - PubMed
-
- Hardy J. & Selkoe D. J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297, 353–356 (2002). - PubMed
-
- Dickson D. W. The pathogenesis of senile plaques. J. Neuropathol. Exp. Neurol. 56, 321–339 (1997). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

