Advanced glycation end product recognition by the receptor for AGEs
- PMID: 21565706
- PMCID: PMC3150472
- DOI: 10.1016/j.str.2011.02.013
Advanced glycation end product recognition by the receptor for AGEs
Abstract
Nonenzymatic protein glycation results in the formation of advanced glycation end products (AGEs) that are implicated in the pathology of diabetes, chronic inflammation, Alzheimer's disease, and cancer. AGEs mediate their effects primarily through a receptor-dependent pathway in which AGEs bind to a specific cell surface associated receptor, the Receptor for AGEs (RAGE). N(ɛ)-carboxy-methyl-lysine (CML) and N(ɛ)-carboxy-ethyl-lysine (CEL), constitute two of the major AGE structures found in tissue and blood plasma, and are physiological ligands of RAGE. The solution structure of a CEL-containing peptide-RAGE V domain complex reveals that the carboxyethyl moiety fits inside a positively charged cavity of the V domain. Peptide backbone atoms make specific contacts with the V domain. The geometry of the bound CEL peptide is compatible with many CML (CEL)-modified sites found in plasma proteins. The structure explains how such patterned ligands as CML (CEL)-proteins bind to RAGE and contribute to RAGE signaling.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

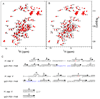
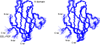
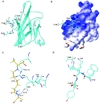
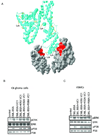
Comment in
-
How to create a specific recognition for an unspecific interaction.Structure. 2011 May 11;19(5):601-2. doi: 10.1016/j.str.2011.04.003. Structure. 2011. PMID: 21565694
References
-
- Atherton E, Fox H, Harkiss D, Sheppard RC. J. Chem. Soc., Chem Commun. 1978:537–539.
-
- Bork P, Holm L, Sander C. The immunoglobulin fold. Structural classification, sequence patterns and common core. J Mol Biol. 1994;242:309–320. - PubMed
-
- Brett J, Schmidt AM, Zou YS, Yan SD, Weidman E, Pinsky D, Neeper M, Przysiecki M, shaw A, Migheli A, Stern D. Tissue distribution of the receptor for advanced glycation end products (RAGE): expression in smooth muscle, cardiac myocyte, and neural tissue in addition to vasculature. Am. J. Pathol. 1993;143:1699–1712. - PMC - PubMed
-
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Annu Rev Med. 1995;46:223–234. - PubMed
-
- Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation and the pathogenesis of diabetic complications. Ann Intern Med. 1984;101:527–537. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

