Cross-reactivity of a rice NLR immune receptor to distinct effectors from the rice blast pathogen Magnaporthe oryzae provides partial disease resistance
- PMID: 31296569
- PMCID: PMC6721932
- DOI: 10.1074/jbc.RA119.007730
Cross-reactivity of a rice NLR immune receptor to distinct effectors from the rice blast pathogen Magnaporthe oryzae provides partial disease resistance
Abstract
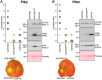
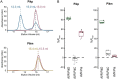
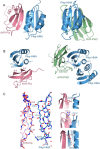
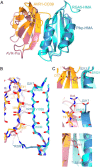
Unconventional integrated domains in plant intracellular immune receptors of the nucleotide-binding leucine-rich repeat (NLRs) type can directly bind translocated effector proteins from pathogens and thereby initiate an immune response. The rice (Oryza sativa) immune receptor pairs Pik-1/Pik-2 and RGA5/RGA4 both use integrated heavy metal-associated (HMA) domains to bind the effectors AVR-Pik and AVR-Pia, respectively, from the rice blast fungal pathogen Magnaporthe oryzae These effectors both belong to the MAX effector family and share a core structural fold, despite being divergent in sequence. How integrated domains in NLRs maintain specificity of effector recognition, even of structurally similar effectors, has implications for understanding plant immune receptor evolution and function. Here, using plant cell death and pathogenicity assays and protein-protein interaction analyses, we show that the rice NLR pair Pikp-1/Pikp-2 triggers an immune response leading to partial disease resistance toward the "mis-matched" effector AVR-Pia in planta and that the Pikp-HMA domain binds AVR-Pia in vitro We observed that the HMA domain from another Pik-1 allele, Pikm, cannot bind AVR-Pia, and it does not trigger a plant response. The crystal structure of Pikp-HMA bound to AVR-Pia at 1.9 Å resolution revealed a binding interface different from those formed with AVR-Pik effectors, suggesting plasticity in integrated domain-effector interactions. The results of our work indicate that a single NLR immune receptor can bait multiple pathogen effectors via an integrated domain, insights that may enable engineering plant immune receptors with extended disease resistance profiles.
Keywords: Nod-like receptor (NLR); effector; host-pathogen interaction; plant biochemistry; plant defense; plant immunity; protein structure; rice; rice blast disease.
© 2019 Varden et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

