Solution structures of a DNA dodecamer duplex with and without a cisplatin 1,2-d(GG) intrastrand cross-link: comparison with the same DNA duplex containing an oxaliplatin 1,2-d(GG) intrastrand cross-link
- PMID: 17497831
- PMCID: PMC2129171
- DOI: 10.1021/bi062291f
Solution structures of a DNA dodecamer duplex with and without a cisplatin 1,2-d(GG) intrastrand cross-link: comparison with the same DNA duplex containing an oxaliplatin 1,2-d(GG) intrastrand cross-link
Abstract
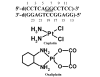
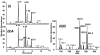
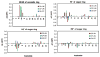
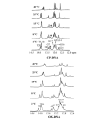
Proteins that discriminate between cisplatin-DNA adducts and oxaliplatin-DNA adducts are thought to be responsible for the differences in tumor range, toxicity, and mutagenicity of these two important chemotherapeutic agents. However, the structural basis for differential protein recognition of these adducts has not been determined and could be important for the design of more effective platinum anticancer agents. We have determined high-resolution NMR structures for cisplatin-GG and undamaged DNA dodecamers in the AGGC sequence context and have compared these structures with the oxaliplatin-GG structure in the same sequence context determined previously in our laboratory. This structural study allows the first direct comparison of cisplatin-GG DNA and oxaliplatin-GG DNA solution structures referenced to undamaged DNA in the same sequence context. Non-hydrogen atom rmsds of 0.81 and 1.21 were determined for the 15 lowest-energy structures for cisplatin-GG DNA and undamaged DNA, respectively, indicating good structural convergence. The theoretical NOESY spectra obtained by back-calculation from the final average structures showed excellent agreement with the experimental data, indicating that the final structures are consistent with the NMR data. Several significant conformational differences were observed between the cisplatin-GG adduct and the oxaliplatin-GG adduct, including buckle at the 5' G6.C19 base pair, opening at the 3' G7.C18 base pair, twist at the A5G6.T20C19 base pair step, slide, twist, and roll at the G6G7.C19C18 base pair step, slide at the G7C8.C18G17 base pair step, G6G7 dihedral angle, and overall bend angle. We hypothesize that these conformational differences may be related to the ability of various DNA repair proteins, DNA binding proteins, and DNA polymerases to discriminate between cisplatin-GG and oxaliplatin-GG adducts.
Figures



References
-
- Greene MH. Is Cisplatin a Human Carcinogen. J Natl Cancer Inst. 1992;84:306–312. - PubMed
-
- Travis LB, Curtis RE, Storm H, Hall P, Holowaty E, Van Leeuwen FE, Kohler BA, Pukkala E, Lynch CF, Andersson M, Bergfeldt K, Clarke EA, Wiklund T, Stoter G, Gospodarowicz M, Sturgeon J, Fraumeni JF, Jr, Boice JD., Jr Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429–1439. - PubMed
-
- Silva MJ, Costa P, Dias A, Valente M, Louro H, Boavida MG. Comparative analysis of the mutagenic activity of oxaliplatin and cisplatin in the Hprt gene of CHO cells. Environ Mol Mutagen. 2005;46:104–115. - PubMed
-
- Bassett E, King NM, Bryant MF, Hector S, Pendyala L, Chaney SG, Cordeiro-Stone M. The role of DNA polymerase η in translesion synthesis past platinum-DNA adducts in human fibroblasts. Cancer Res. 2004;64:6469–6475. - PubMed
-
- Page JD, Husain I, Sancar A, Chaney SG. Effect of the diaminocyclohexane carrier ligand on platinum adduct formation, repair, and lethality. Biochemistry. 1990;29:1016–1024. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

