Crystal structures of a quorum-quenching antibody
- PMID: 17400249
- PMCID: PMC1994716
- DOI: 10.1016/j.jmb.2007.02.081
Crystal structures of a quorum-quenching antibody
Abstract
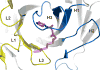
A large number of Gram-negative bacteria employ N-acyl homoserine lactones (AHLs) as signaling molecules in quorum sensing, which is a population density-dependent mechanism to coordinate gene expression. Antibody RS2-1G9 was elicited against a lactam mimetic of the N-acyl homoserine lactone and represents the only reported monoclonal antibody that recognizes the naturally-occuring N-acyl homoserine lactone with high affinity. Due to its high cross-reactivity, RS2-1G9 showed remarkable inhibition of quorum sensing signaling in Pseudomonas aeruginosa, a common opportunistic pathogen in humans. The crystal structure of Fab RS2-1G9 in complex with a lactam analog revealed complete encapsulation of the polar lactam moiety in the antibody-combining site. This mode of recognition provides an elegant immunological solution for tight binding to an aliphatic, lipid-like ligand with a small head group lacking typical haptenic features, such as aromaticity or charge, which are often incorporated into hapten design to generate high-affinity antibodies. The ability of RS2-1G9 to discriminate between closely related AHLs is conferred by six hydrogen bonds to the ligand. Conversely, cross-reactivity of RS2-1G9 towards the lactone is likely to originate from conservation of these hydrogen bonds as well as an additional hydrogen bond to the oxygen of the lactone ring. A short, narrow tunnel exiting at the protein surface harbors a portion of the acyl chain and would not allow entry of the head group. The crystal structure of the antibody without its cognate lactam or lactone ligands revealed a considerably altered antibody-combining site with a closed binding pocket. Curiously, a completely buried ethylene glycol molecule mimics the lactam ring and, thus, serves as a surrogate ligand. The detailed structural delineation of this quorum-quenching antibody will aid further development of an antibody-based therapy against bacterial pathogens by interference with quorum sensing.
Figures






References
-
- Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. - PubMed
-
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. - PubMed
-
- Smith RS, Iglewski BH. P. aeruginosa quorum-sensing systems and virulence. Curr Opin Microbiol. 2003;6:56–60. - PubMed
-
- Kravchenko VV, Kaufmann GF, Mathison JC, Scott DA, Katz AZ, Wood MR, Brogan AP, Lehmann M, Mee JM, Iwata K, Pan Q, Fearns C, Knaus UG, Meijler MM, Janda KD, Ulevitch RJ. N-(3-oxo-acyl)homoserine lactones signal cell activation through a mechanism distinct from the canonical pathogen-associated molecular pattern recognition receptor pathways. J Biol Chem. 2006;281:28822–28830. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

