Structure of 6-diazo-5-oxo-norleucine-bound human gamma-glutamyl transpeptidase 1, a novel mechanism of inactivation
- PMID: 28378915
- PMCID: PMC5441403
- DOI: 10.1002/pro.3172
Structure of 6-diazo-5-oxo-norleucine-bound human gamma-glutamyl transpeptidase 1, a novel mechanism of inactivation
Abstract
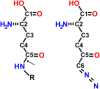
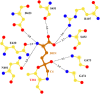
Intense efforts are underway to identify inhibitors of the enzyme gamma-glutamyl transpeptidase 1 (GGT1) which cleaves extracellular gamma-glutamyl compounds and contributes to the pathology of asthma, reperfusion injury and cancer. The glutamate analog, 6-diazo-5-oxo-norleucine (DON), inhibits GGT1. DON also inhibits many essential glutamine metabolizing enzymes rendering it too toxic for use in the clinic as a GGT1 inhibitor. We investigated the molecular mechanism of human GGT1 (hGGT1) inhibition by DON to determine possible strategies for increasing its specificity for hGGT1. DON is an irreversible inhibitor of hGGT1. The second order rate constant of inactivation was 0.052 mM-1 min-1 and the Ki was 2.7 ± 0.7 mM. The crystal structure of DON-inactivated hGGT1 contained a molecule of DON without the diazo-nitrogen atoms in the active site. The overall structure of the hGGT1-DON complex resembled the structure of the apo-enzyme; however, shifts were detected in the loop forming the oxyanion hole and elements of the main chain that form the entrance to the active site. The structure of hGGT1-DON complex revealed two covalent bonds between the enzyme and inhibitor which were part of a six membered ring. The ring included the OG atom of Thr381, the reactive nucleophile of hGGT1 and the α-amine of Thr381. The structure of DON-bound hGGT1 has led to the discovery of a new mechanism of inactivation by DON that differs from its inactivation of other glutamine metabolizing enzymes, and insight into the activation of the catalytic nucleophile that initiates the hGGT1 reaction.
Keywords: crystal structure; enzyme inactivation; enzyme kinetics; gamma-glutamyl transferase; human gamma-glutamyl transpeptidase; protein conformation.
© 2017 The Protein Society.
Figures




References
-
- Yamamoto S, Watanabe B, Hiratake J, Tanaka R, Ohkita M, Matsumura Y (2011) Preventive effect of ggstop, a novel and selective gamma‐glutamyl transpeptidase inhibitor, on ischemia/reperfusion‐induced renal injury in rats. J Pharmacol Exp Ther 339:945–951. - PubMed
-
- Terzyan SS, Burgett AW, Heroux A, Smith CA, Mooers BH, Hanigan MH (2015) Human gamma‐glutamyl transpeptidase 1: structures of the free enzyme, inhibitor‐bound tetrahedral transition states, and glutamate‐bound enzyme reveal novel movement within the active site during catalysis. J Biol Chem 290:17576–17586. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

