Structural and thermodynamic consequences of 1-(4-chlorophenyl)imidazole binding to cytochrome P450 2B4
- PMID: 17887776
- PMCID: PMC2566820
- DOI: 10.1021/bi7011614
Structural and thermodynamic consequences of 1-(4-chlorophenyl)imidazole binding to cytochrome P450 2B4
Abstract
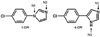
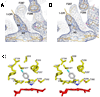
The crystal structure of P450 2B4 bound with 1-(4-chlorophenyl)imidazole (1-CPI) has been determined to delineate the structural basis for the observed differences in binding affinity and thermodynamics relative to 4-(4-chlorophenyl)imidazole (4-CPI). Compared with the previously reported 4-CPI complex, there is a shift in the 1-CPI complex of the protein backbone in helices F and I, repositioning the side chains of Phe-206, Phe-297, and Glu-301, and leading to significant reshaping of the active site. Phe-206 and Phe-297 exchange positions, with Phe-206 becoming a ligand-contact residue, while Glu-301, rather than hydrogen bonding to the ligand, flips away from the active site and interacts with His-172. As a result the active site volume expands from 200 A3 in the 4-CPI complex to 280 A3 in the 1-CPI complex. Based on the two structures, it was predicted that a Phe-206-->Ala substitution would alter 1-CPI but not 4-CPI binding. Isothermal titration calorimetry experiments indicated that this substitution had no effect on the thermodynamic signature of 4-CPI binding to 2B4. In contrast, relative to wild-type 1-CPI binding to F206A showed significantly less favorable entropy but more favorable enthalpy. This result is consistent with loss of the aromatic side chain and possible ordering of water molecules, now able to interact with Glu-301 and exposed residues in the I-helix. Hence, thermodynamic measurements support the active site rearrangement observed in the crystal structure of the 1-CPI complex and illustrate the malleability of the active site with the fine-tuning of residue orientations and thermodynamic signatures.
Figures


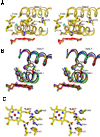

References
-
- Rendic S. Summary of information on human CYP enzymes: Human P450 metabolism data. Drug Metabolism Reviews. 2002;34:83–448. - PubMed
-
- Zhao Y, Halpert JR. Structure-function analysis of cytochromes P450 2B. Biochim. Biophys. Acta. 2007;1770:402–412. - PubMed
-
- Johnson EF, Stout CD. Structural diversity of human xenobiotic-metabolizing cytochrome P450 monooxygenases. Biochem. Biophys. Res. Commun. 2005;338:331–336. - PubMed
-
- Poulos TL. Structural and functional diversity in heme monooxygenases. Drug Metab. Dispos. 2005;33:10–18. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

