Autotransporter structure reveals intra-barrel cleavage followed by conformational changes
- PMID: 17994105
- PMCID: PMC2551741
- DOI: 10.1038/nsmb1322
Autotransporter structure reveals intra-barrel cleavage followed by conformational changes
Abstract
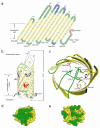
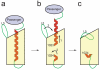
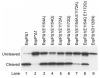
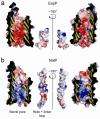
Autotransporters are virulence factors produced by Gram-negative bacteria. They consist of two domains, an N-terminal 'passenger' domain and a C-terminal beta-domain. beta-domains form beta-barrel structures in the outer membrane while passenger domains are translocated into the extracellular space. In some autotransporters, the two domains are separated by proteolytic cleavage. Using X-ray crystallography, we solved the 2.7-A structure of the post-cleavage state of the beta-domain of EspP, an autotransporter produced by Escherichia coli strain O157:H7. The structure consists of a 12-stranded beta-barrel with the passenger domain-beta-domain cleavage junction located inside the barrel pore, approximately midway between the extracellular and periplasmic surfaces of the outer membrane. The structure reveals an unprecedented intra-barrel cleavage mechanism and suggests that two conformational changes occur in the beta-domain after cleavage, one conferring increased stability on the beta-domain and another restricting access to the barrel pore.
Figures


References
-
- Jacob-Dubuisson F, Fernandez R, Coutte L. Protein secretion through autotransporter and two-partner pathways. Biochim Biophys Acta. 2004;1694:235–57. - PubMed
-
- Thanassi DG, Stathopoulos C, Karkal A, Li H. Protein secretion in the absence of ATP: the autotransporter, two-partner secretion and chaperone/usher pathways of gram-negative bacteria (review) Mol Membr Biol. 2005;22:63–72. - PubMed
-
- Cotter SE, Surana NK, Geme JW., 3rd Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 2005;13:199–205. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

